Level position&phosphor performance
Occupied states: that can donate electrons.
Empty states: that can accept electrons.

- (a) the downward shift of the lowest-energy 5d level when a lanthanide is brought from the gaseous state (free ion) into the crystalline environment of a compound (A).
- (b) the importance of lowest-energy 5d level location relative to 4f2 levels in Pr3+. 如果5d能级的位置合适,就可能有cascade emission of two photons,也就是量子效率大于100 %。
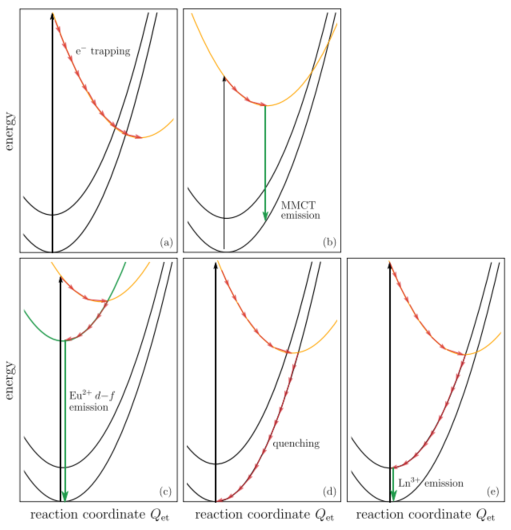
另外,Depending on the precise location of the lowest 5d state in Nd3+, Eu2+, and Sm2+, either broad-band 5d–4f or narrowline 4f–4f emissions can be observed. - (c)(d)(e) interplay between the localized 5d electron and the delocalized conduction band states.
(c) 中autoionization occurs spontaneously and no 5d–4f emission is observed. 例子LaAlO3:Ce3+, rare-earth sesquioxides Ln2O3:Ce3+, and also for Eu2+ on trivalent rare-earth sites in oxide compounds.
(d) The 5d electron delocalizes but remains in the vicinity of the hole left behind. The true nature of the state, which is sometimes called an impurity trapped exciton state, is not precisely known. The recombination of the electron with the hole leads to the so-called anomalous emission characterized by a very large Stokes shift.
(e) 5d state well below the conduction band, leading to 5d–4f emission. The thermal quenching of this emission by means of ionization to conduction band states is controlled by the energy EdC. - (f) a typical situation for Pr3+ in a transition metal complex compound like CaTiO3. The undesired blue emission from the Pr3+ 3P0 level is quenched by intervalence charge transfer (IVCT). The electron transfers from the 3P0 level to the transition metal (Ti4+). The electron is transferred back to the red emitting Pr3+ 1D4 level. The position of the 3P0 level relative to the transition metal-derived conduction band controls the quenching process, and thereby the color of emission.
- 上面讨论的是稀土离子的 occupied states,实际上三价稀土离子may accept an electron to form a divalent lanthanide ion. The location of the occupied ground-state level of a divalent lanthanide ion is therefore the same as the unoccupied electron-accepting state of the corresponding trivalent lanthanide ion.
- The accepted electron may originate from the valence band, the conduction band, or another lanthanide ion.
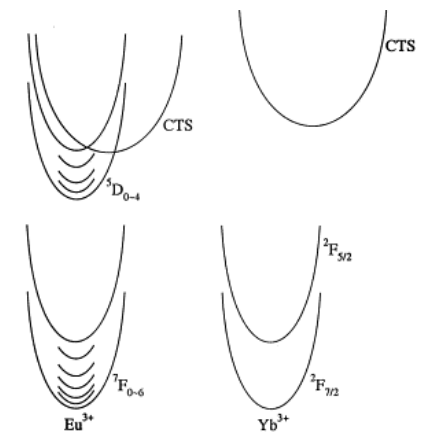
- (g) The excitation of an electron from the valence band to the unoccupied state creates the ground state of Eu2+. This is a dipole allowed transition that is used, for example, to sensitize Y2O3:Eu3+ phosphors to the 254 nm Hg emission in tube lighting. Recombination of the electron with the valence band hole leaves the Eu3+ ion in the 5D0 excited state resulting in red 4f6–4f6 emission. (下图中箭头3表示Eu-O电荷迁移态,从价带顶到Eu2+的基态能级,参考这里)
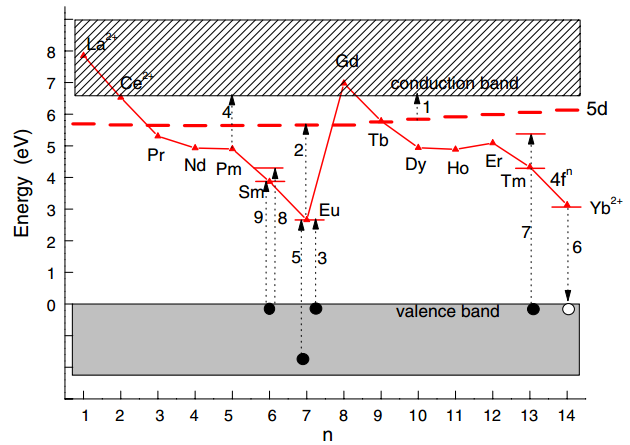
- (h) In the case of Yb3+ the recombination with the hole in the valence band produces a strong Stokes-shifted charge transfer (CT) luminescence. This type of luminescence gained considerable interest for developing scintillators for neutrino detection.
- (i) the trapping of an electron from the conduction band by Sm3+ to form the ground state of Sm2+. The absolute location of an “unoccupied” divalent lanthanide ground state determines the electron trapping depth provided by the corresponding trivalent lanthanide ion. Sm2+的基态能级到价带顶的距离决定了trap depth。
- (j) trapping of a hole from the valence band by Ce3+. This hole trapping is an important aspect of the scintillation mechanism in Ce3+- doped scintillators. Similarly, Eu2+ is an efficient hole trap of importance for the X-ray storage phosphor BaFBr:Eu2+. The absolute location of an “occupied” trivalent lanthanide ground state determines the valence band hole trapping depth provided by that lanthanide ion. (下图来自Dorenbos-JMCC-2018)
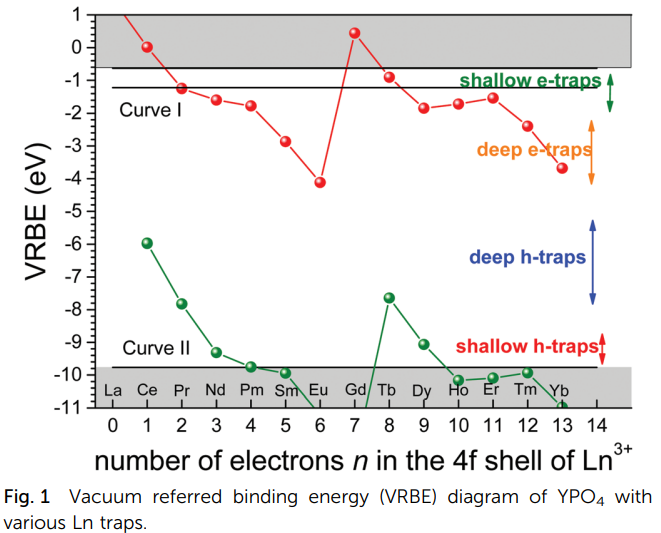
- (k) (l) Phosphor properties become more complicated when we deal with “double lanthanide-doped systems.”
(k) the situation in Eu2+ and Sm3+ double-doped compounds like SrS and MgS that were studied for optical data storage applications. 用ultraviolet写入信息,得到Eu3+和Sm2+,然后用infrared read pulse liberates the electron again from Sm2+, resulting, eventually, in Eu2+ 5d–4f emission.
类似的例子还有 Y2SiO5:Ce3+;Sm3+ and LiYSiO4:Ce3+;Sm3+ for X-ray and thermal neutron storage phosphor applications, respectively.
(l) The Ce3+ electron excited to the lowest 5d state can jump to Eu3+ when the unoccupied Eu2+ ground state is located at a lower energy than the occupied lowest Ce3+ 5d excited state. After the jump, Eu2+ and Ce4+ are formed. The Eu2+ electron can jump back to Ce4+ if the unoccupied Ce3+ ground state is located below the occupied Eu2+ ground state. The original situation is restored without emission of a photon. Similar quenching routes pertain to Ce3+ in Yb-based compounds, and with appropriate level schemes, other “killing” combinations can be found as well. - 注意:The true mechanism in the persistent luminescence phosphor SrAl2O4:Eu2+;Dy3+ is still disputed. One needs to know the absolute level energy locations to arrive at plausible mechanisms or to discard implausible ones.
以上内容来自Phosphor Handbook,下面结合我自己看的文献实例进一步探讨上面的几种情况。
Charge Transfer之旅
LMCT/MLCT
MLCT在氧化物荧光粉中不太可能发生,但是在配合物中比较常见;我们主要讨论的荧光粉涉及的都是LMCT,下面介绍几个典型的LMCT。[固体发光材料—P76]
Eu3+和配位离子(O/F/Cl/Br/I/S)的电荷迁移态
- Eu3+-O2-电荷迁移态在不同基质中的位置,参考[Dorenbos-The Eu3+ charge transfer energy and the relation with the band gap of compounds-2005]以及[The charge-transfer absorption band of Eu3+ in oxides-JSSC-1975]
- 通过Eu3+和配体的CT数据估算其他三价稀土离子的CT位置:Dorenbos presented a theoretical model that allows estimation of the charge-transfer energy of any lanthanide ions from the knowledge of the position of the charge-transfer band of Eu3+. [P. Dorenbos-ECS-2013] [Interpretation of europium(III) spectra-Koen Binnemans-Coordination Chemistry Reviews-2015]
- CaF2:Eu3+的特殊性:CaF2:Eu3+ is a rare example of a europium(III) compound in which both the f–d transitions and the charge-transfer band are observed. [The europium(III)—fluorine charge-transfer transition-1989]
- CT和f-d跃迁的对比:f-d跃迁和CT跃迁都是opposite-parity transition,但是后者更broader,而且是structureless。 [The europium(III)—fluorine charge-transfer transition-1989]
- 典型的氟化物和氧化物中CT:3:Eu3+中的电荷迁移带在150 nm,而Y2O3:Eu3+在254 nm,电子从F–迁移到Eu3+要比从O2-到Eu3+更消耗能量。
- CT对寿命的影响:The lower position of the Eu3+-O2- CT band increases the oscillator strength of the forced-electricdipole transitions, which in turn reduces the lifetime of the excited state in an impressive way. [The europium(III)—fluorine charge-transfer transition-1989] [Interpretation of europium(III) spectra-Koen Binnemans-Coordination Chemistry Reviews-2015]
Yb3+的电荷迁移态发光
Ce4+的电荷迁移态发光
Cerium is a rare earth element, which presents two stable oxidation states: Ce4+ and Ce3+. Ce4+ displays a [Xe]4f0 electronic configuration, and is a non-luminescent center, except in the case of O2-→Ce4+ charge transfer, while Ce3+ is a well-known luminescent center, with a [Xe]4f1 electronic configuration.[Tuning the oxidation states of dopants: a strategy for the modulation of material photoluminescence properties-Chemistry–A European Journal, 2021]
[Sr2CeO4-PCCP-2016] [SrZnO2:Ce-PL-2021] [On the Nature of the Luminescence of Sr2CeO4-A. Meijerink-jes-2000]
IVCT和MMCT的定义和经典例子
IVCT: Electron transfer (thermal or photoinduced) between two metal sites differing only in oxidation state. Quite often such electron transfer reverses the oxidation states of the sites. The term is frequently extended to the case of metal-to-metal charge transfer between non-equivalent metal centres. [IPUAC]
The transition produces a characteristically intense absorption in the electromagnetic spectrum. The band is usually found in the visible or near infrared region of the spectrum and is broad.
MMCT: An electronic transition of a bi- or poly-nuclear metal complex that corresponds to excitation populating an electronic state in which considerable electron transfer between two metal centres has occurred. [IUPAC]
例子:
(1) 【普鲁士蓝】(Prussian blue),\(\mathrm{Fe}_4{ }^{\mathrm{III}}\left[\mathrm{Fe}^{\mathrm{II}}(\mathrm{CN})_6\right]_3\) (参考wiki)
颜色来源:The intense blue color of Prussian blue is associated with the energy of the transfer of electrons from Fe(II) to Fe(III). Many such mixed-valence compounds absorb certain wavelengths of visible light resulting from intervalence charge transfer. In this case, orange-red light around 680 nm in wavelength is absorbed, and the reflected light appears blue as a result.
准确的颜色:Prussian blue is strongly colored and tends towards black and dark blue when mixed into oil paints. The exact hue depends on the method of preparation, which dictates the particle size.
显示器显示:Like most high-chroma pigments, Prussian blue cannot be accurately displayed on a computer display.
光致变色:PB is electrochromic—changing from blue to colorless upon reduction. This change is caused by reduction of the Fe(III) to Fe(II), eliminating the intervalence charge transfer that causes Prussian blue's color.
(2) 蓝宝石
xx
参考文献:
[Microscopic origin of the optical processes in blue sapphire-CC-2013] [Effect of Reduction Annealing on the Coloration Mechanism of Yellow Sapphire with High Iron Content-Crystal-2022] [Metal-to-Metal Charge Transfer in AWO4 (A = Mg, Mn, Co, Ni, Cu, or Zn) Compounds with the Wolframite Structure]稀土-稀土-MMCT Quenching
Ce4+-Eu2+ MMCT state was held responsible for the mutual emission quenching for Ce3+ and Eu3+. 作者之前报道了Ce3+-(Gd3+)n-Tb3+的energy transport type,进一步地,作者发现可以实现Ce3+-(Gd3+)n-Eu3+的高效能量传递,避免了之前MMCT导致的mutual emission quenching。[Energy Transfer from Ce3+ to Eu3+ in (Y, Gd)F3-1983-G. Blasse]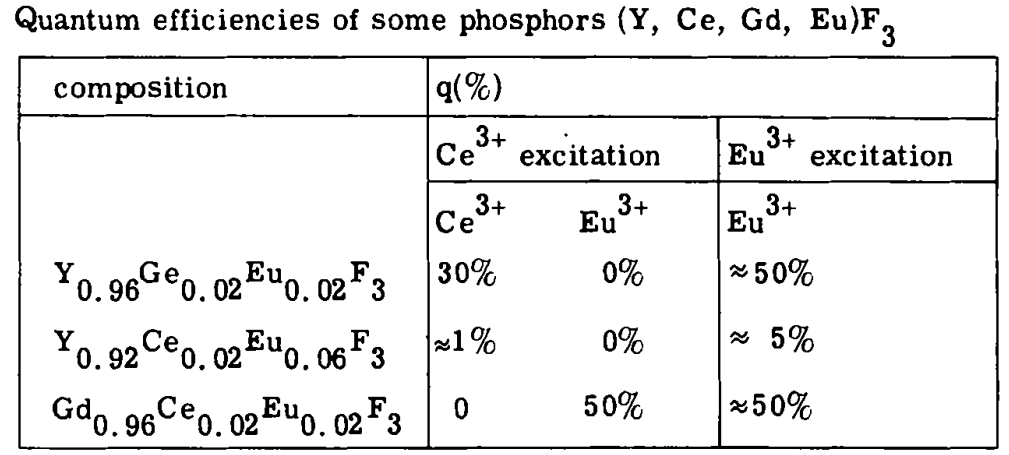
bridge ions除了Gd3+,也可以是Tb3+,实现Ce3+-(Tb3+)n-Eu3+的能量传递。[Sensitizing Eu3+ with Ce3+ and Tb3+ to Make Narrow-Line Red Phosphors for Light Emitting Diodes]
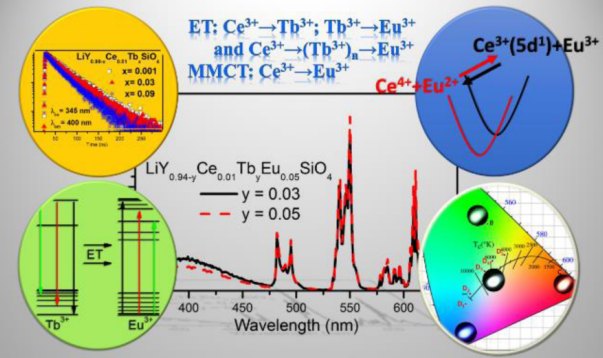 上图参考[Consequences of ET and MMCT on Luminescence of Ce3+-, Eu3+-, and Tb3+-doped LiYSiO4-IC-2016]
上图参考[Consequences of ET and MMCT on Luminescence of Ce3+-, Eu3+-, and Tb3+-doped LiYSiO4-IC-2016]

It is well known that this approach suffers from metal-to-metal charge transfer (MMCT) quenching between Ce3+ and Eu3+. To reduce this quenching mechanism in Tb3Al5O12 (TAG), the sensitizer and activator were spatially separated by creating core-shell particles. [Thomas Jüstel-JL-2018]
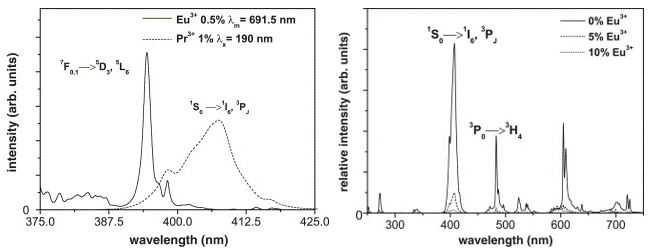
Pr4+-Eu2+ MMCT,由于这个电荷迁移态能量比较低,所以激发Pr3+到\(\mathrm{~^1S}_{0}\)能级,之后并没有将能量传递给Eu3+, 反而Pr3+的该能级的发光被猝灭了。Pr3+-Eu3+ 之间虽然存在favorable spectral overlap for energy transfer,但是并没有观测到能量传递,反而是Pr3+的发光被猝灭了。Pr4+-Yb2+ MMCT的存在也会导致Pr3+-Yb3+共掺杂的样品出现类似的现象。[Meijerink-2005-JL]
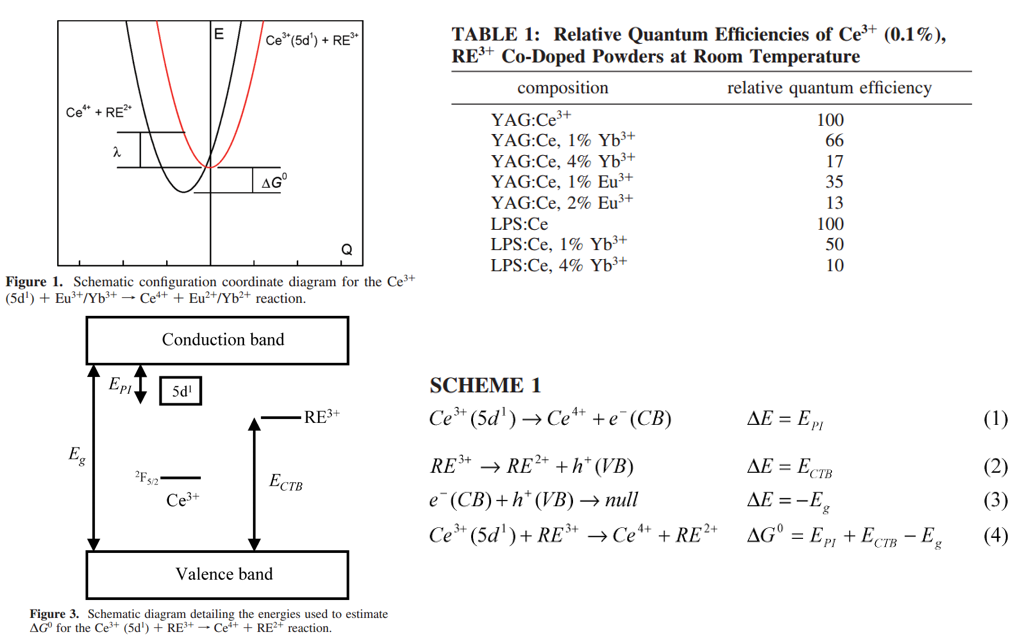
Ce4+-Eu2+ 或Ce4+-Yb2+ MMCT,Standard semiclassical, high-temperature models for electron transfer are shown to be able to quantitatively describe the room-temperature electron transfer reactions between Ce3+(5d1) donors and Eu3+/Yb3+ acceptors in insulating oxide hosts. 共掺杂Eu3+/Yb3+导致Ce3+的发光被猝灭,注意这里Ce3+到Eu3+/Yb3+的能量传递很弱或者可以被忽略,具体内容在Marcus theory那个章节有详细说明。[Quantitative Analysis of Electron Transfer-JPCC-2010-Anant Setlur]
有的基质中,借助Ce4+-Yb2+ MMCT可以实现Ce3+到Yb3+的能量,比如文献[Spectral Properties and Energy Transfer between Ce3+ and Yb3+ in the Ca3Sc2Si3O12 Host: Is It an Electron Transfer Mechanism?-JPCA-2016],还有禹德朝的[Insights into the energy transfer mechanism in Ce3+ − Yb3+ codoped YAG phosphors-PRB-2014]
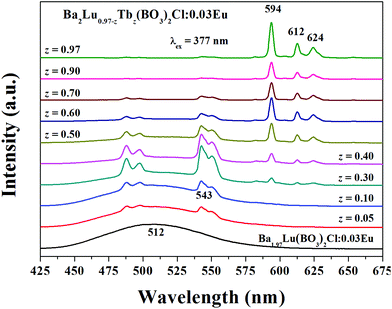 另外,Jia yongchao在[Utilizing Tb3+ as an energy transfer bridge to connect Eu2+–Sm3+ luminescent centers: realization of efficient Sm3+ red emission under near-UV excitation-CC-2012]说Eu2+-Sm3+存在MMCT quenching,所以能量传递不明显,但是引入中间离子Tb3+之后,能量传递就明显了,我对此存疑,没有其他文献有类似报道。
另外,Jia yongchao在[Utilizing Tb3+ as an energy transfer bridge to connect Eu2+–Sm3+ luminescent centers: realization of efficient Sm3+ red emission under near-UV excitation-CC-2012]说Eu2+-Sm3+存在MMCT quenching,所以能量传递不明显,但是引入中间离子Tb3+之后,能量传递就明显了,我对此存疑,没有其他文献有类似报道。
稀土-过渡金属-MMCT Quenching
YVO4:Tb3+一点都不发光,但是在YVO4:Eu3+确是one of the most efficient rare-earthactivated phosphors。These effects have been related to the fact that the charge-transfer state V4+ + Tb4+ is situated at relatively low energy due to the half-filled 4f shell of Tb4+ (4f7).
参考[Strong Quenching of Tb3+ Emission by Tb–V Interaction in YPO4–YVO4-JCP-1970]以及[Energy transfer from vanadate to rare-earth ions in calcium sulphate-PSS-1974]
Tb3+-to-As5+ quenching:YVxAs1−xO4 Solid-State Solution 参考[Emission Quenching and First Evidence of Tb3+-to-As5+ Charge Transfer in Terbium(III) Ion-Doped YVxAs1−xO4 Solid-State Solution—JPCC-2020]
在最近的文章中,[The Influence of Defects on the Luminescence of Trivalent Terbium in Nanocrystalline Yttrium Orthovanadate-Nano Letter-2022],作者实现了YVO4:Tb3+的发光,文章要点如下:
- Different works proposed that the quenching rates are reduced when MMCT states are shifted to higher energies, which could be achieved by increasing the positive charge density on the Tb3+ sites or increasing the electron density of the transition metal center. This has been confirmed by the observation of VO43− → Tb3+ energy transfer in aliovalent-substituted CaSO4:V5+,Tb3+.
([Energy transfer from vanadate to rare-earth ions in calcium sulphate-PSS-1974] In calcium sulphate the V5+ ion occupies a S6+ site, i.e.. it bears an effectively negative charge, whereas the Tb3+ occupying a Cazt site bears an effectively positive charge. To attain the charge-transfer state, V4+ + Tb4+, negative charge has to be transferred from a positively charged site to a negatively charged site. It is obvious that this process will take considerably more energy in calcium sulphate than, for exa,mple, in YVO4-:Tb where the relevant centres do not bear effective charges.) - (Y1−xTbx)VO4 nanoparticles obtained by colloidal precipitation showed expanded unit cells, high defect densities, and intimately mixed carbonates and hydroxides, which contribute to a shift of the MMCT states to higher energies. Consequently, we demonstrate unambiguously for the first time that Tb3+ luminescence can be excited by VO43− → Tb3+ energy transfer

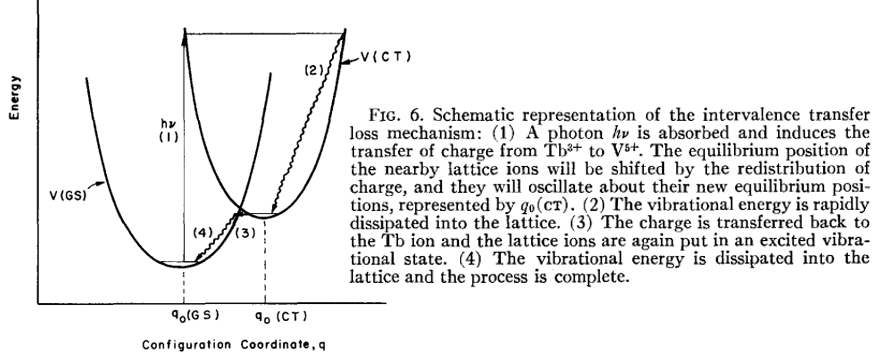
A novel thermometric strategy utilizing IVCT interfered Pr3+ emission is proposed. 参考[Intervalence charge transfer state interfered Pr3+ luminescence: A novel strategy for high sensitive optical thermometry-Sensors and Actuators B: Chemical-2017]
MMCT能量传递
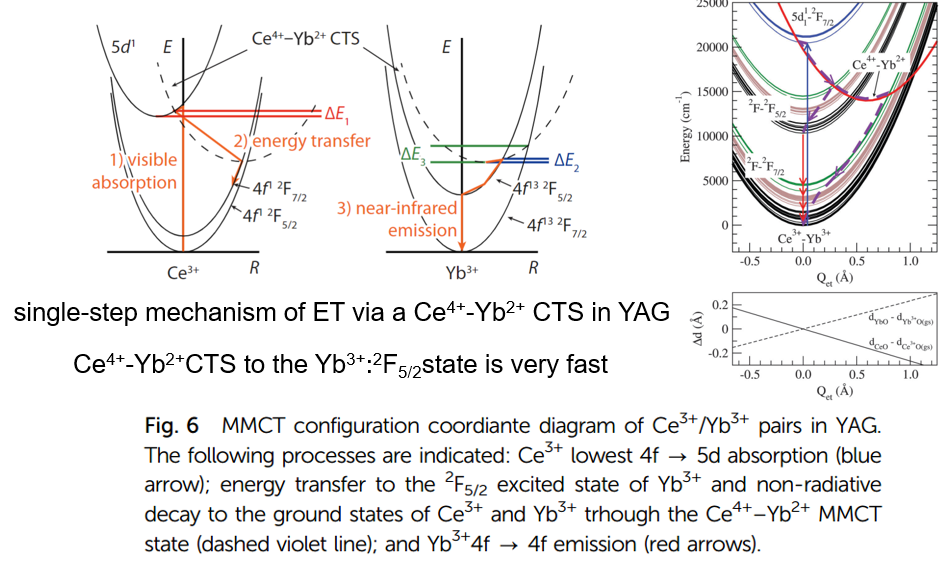
上图别分来自禹德朝的[Insights into the energy transfer mechanism in Ce3+ − Yb3+ codoped YAG phosphors-PRB-2014]和[Zoila Barandiara´n-PCCP-2015]
文献[Spectral Properties and Energy Transfer between Ce3+ and Yb3+ in the Ca3Sc2Si3O12 Host: Is It an Electron Transfer Mechanism?-JPCA-2016]也报道了Ce3+到Yb3+的能量传递。
MMCT 能量存储(YPO4:Ce,Sm)
This is a very interesting host (YPO4:Ce, Ln) where Dorenbos showed that the trend of predicted trap depths agrees very well with the trend of experimentally determined levels. For YPO4:Ce, Sm, there exist two main TL bands. The following picture are the TL after gama irradiation. The low-temperature band corresponds to the emission from Sm3+ while the high-temperature band can be assigned to the emission from Ce3+.
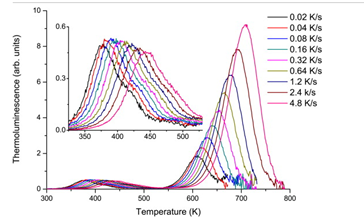 The Sm3+ behave as expected from Randall–Wilkins model: as the heating rate increases, the glow-peak maxima shifts to higher temperature and the peak maxima decrease. However, the peak area (proportional to the dose) remains constant. While this is not the case for the high-temperature band for Ce3+, and for this temperature, Ce3+ intensity should decrease allowing for the thermal quenching effect.
The Sm3+ behave as expected from Randall–Wilkins model: as the heating rate increases, the glow-peak maxima shifts to higher temperature and the peak maxima decrease. However, the peak area (proportional to the dose) remains constant. While this is not the case for the high-temperature band for Ce3+, and for this temperature, Ce3+ intensity should decrease allowing for the thermal quenching effect.
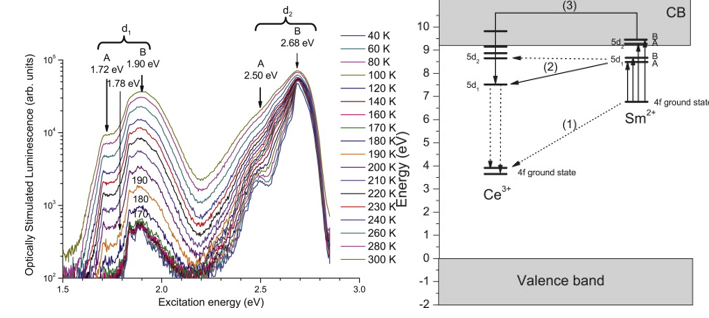
Upon irradiation, the hole from the valence band is captured by Ce3+, which becomes Ce4+, and the electron from the conduction band is captured by Sm3+ becoming Sm2+. Upon stimulation, recombination take place by transport of the electron to Ce4+, which results in an excited 5d1 state of Ce3+; this then relaxes to the ground state yielding the characteristic Ce3+ emission. The possible recombination pathways can be distinguished. Pathway (1) is a centre-to-centre recombination, e.g. a recombination not via the conduction band. The mechanism may be by tunnelling. The energy-level diagram reveals that the recombination must be non-radiative since after tunnelling the available recombination energy is less than the energy of the first 5d state of Ce3+. Pathway (2) is via the first excited state of Sm2+. The driving force may be by thermally stimulated tunnelling; a process observed in other compounds (Delbecq et al., 1974). Pathway (3) is via the conduction band from the 5d2 state of Sm2+.
继续阅读:Luminescence emission from metastable Sm2+ defects in YPO4: Ce, Sm
MMCT in CaF2: Eu,Sm and SrS: Eu, Sm
以上两种都是photoinduced reversible electron transfer,后者还被叫作“IR stimulable phosphor”。The significance of the reversible photoreactions (这两个荧光粉) is broadly recognized as this mechanism allows for a controllable energy storage (upon the forward reaction) and release (upon the backward reaction) . If the backtransfer results into light emission, it is called optically stimulated luminescence (OSL). Alternatively the backtransfer can be provoked by increasing the temperature in which case the process is called thermoluminescence. [Jonas-2021-PRB]
IVCT的特点、分类和发光
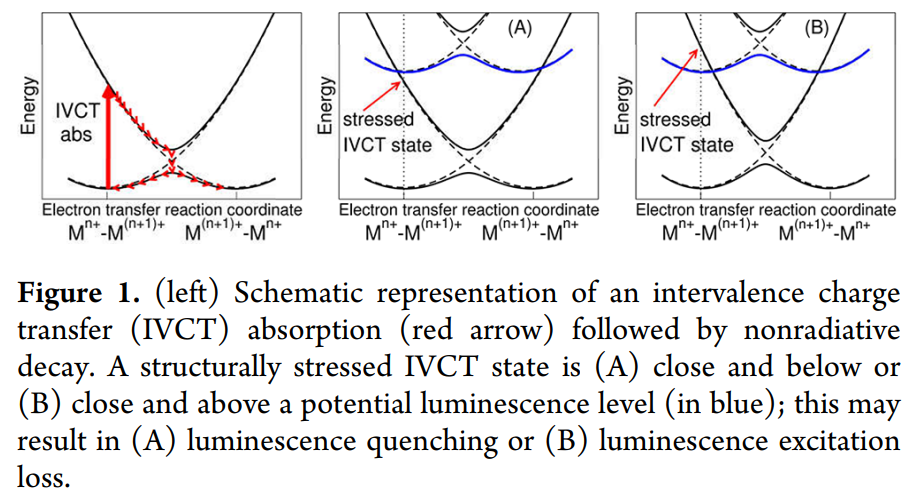 图中的虚线是diabatic energy curves,实线是adiabatic energy curves。
图中的虚线是diabatic energy curves,实线是adiabatic energy curves。
IVCT的特点:
(1) IVCT states produce extremely broad and flat absorption bands due to the large offset between the respective potential energy surfaces (Figure 1, left), which is related to the stressed geometry obtained after the electron transfer. Indeed, Eu2+和Eu3+在配位数相同的情况下离子半径差异较明显,意味着电荷转移后,系统处在stressed的状态,原子核需要有较大移动来达到平衡态。
(2) Furthermore, IVCT absorption is not followed by any emission because the absorbed energy is immediately nonradiatively dissipated by multiphonon relaxation.
以上的characteristics complicate the observation of IVCT states, explaining their absence in the literature.
(3) 存在状态:the presence of dopant pairs or clusters of mixed valence and hence the occurrence of IVCT transitions are expected to have an important impact on the performance of luminescent materials.
IVCT的分类:
(1) case A: IVCT state may result in luminescence quenching in case A, where the crossing between both levels gives rise to an energy barrier for quenching.
(2) case B: IVCT state may result in luminescence excitation loss in case B, where no energy barrier occurs and a full structural relaxation can proceed spontaneously, even at low temperature.
注意上面的两种情况用may这个词是因为如果IVCT state和发光能级的距离足够远(发光能级的最低点离交叉点的energy barrier足够大),才会导致quenching,否则还是会有发光的。以上两种情形是Concerning the effect of IVCT states on regular luminescence levels,如果the stressed IVCT state is far in energy from an emitting level,那么就会出现第三种情况:
(3) When the stressed IVCT state is far in energy from an emitting level, the luminescence is not expected to be quenched, although additional absorption and emission bands can occur.
以上参考[Jonas-JPCL-2019]
IVCT发光
IVCT发光也就是对应于上面的第三种情况(参考[Jonas-JPCL-2019])
(1) anomalous emissions of Ce-doped elpasolites
(2) anomalous emissions of Yb-doped fluorides(CaF2)
在此之前人们一直认为Yb2+的奇异发光来自impurity-trapped excitons。
(3) laser-induced white emission from Ce in Sr2CeO4 [PCCP-2016]
xx
IVCT猝灭发光
BaF2:Eu2+/Eu3+参考[Jonas-JPCL-2019],提到了The crossings between the stressed IVCT states and the excited Eu2+ 4f65d states in BaF2:Eu provide a nonradiative decay channel to the relaxed mixed-valence ground state and an explanation to the fact that the blue Eu2+ 5d−4f emission observed in CaF2 and SrF2, is quenched in BaF2. 这里解释了BaF2中正常Eu2+发光(应该是蓝光)为什么没有观察到,但是并未解释BaF2:Eu2+的abnormal emission。
BaMgAl10O17:Eu [Jonas-JPCL-2019]也提到Nonradiative IVCT decay can hence be a microscopic explanation for concentration quenching. As an example, a study of the blue phosphor BaMgAl10O17:Eu showed that the oxidation of only a small part of Eu2+ has a much more severe effect on the luminescence efficiency than one would expect from the decreasing Eu2+ content.
Eu2+-(Tb3+)n-Eu3+ 另外,Eu2+/Eu3+可能通过IVCT而猝灭,但是Tb3+的引入可能阻碍这种情形,从而实现Eu2+-(Tb3+)n-Eu3+的能量传递。参考[Z. G. Xia, J. Q. Zhuang, A. Meijerink and X. P. Jing, Dalton Trans., 2013, 42, 6327.]
Hydroxyapatite:Yb2+/Yb3+ 参考[Interconfigurational and intraconfigurational transitions of Yb2+ and Yb3+ ions in hydroxyapatite: A cathodoluminescence study-Acta Materialia-2017]
Mnn+-Mn4+
(1) 高掺杂浓度下(10 mol %) K2TiF6: Mn4+,the QE falls below 60%. Luminescence decay curves indicate that the drop in QE can be attributed to an increased probability for direct energy transfer to quenching sites Quenching site (e.g., defects, impurity ions, Mn2+, and Mn3+), the concentration of which increases with the Mn4+ concentration.
(2) In addition, Mn in different valence states (Mn2+ and Mn3+) may be incorporated at higher Mn4+ concentrations. Even if a very small fraction of Mn4+ ions has a different valence state than 4+, effective quenching can occur via metal-to-metal charge-transfer states or direct energy transfer.
(3) 结论部分作者有没有提电荷转移,K2TiF6:Mn4+ decreases due to enhanced direct energy transfer from Mn4+ to quenching sites. Concentration quenching by Mn4+–Mn4+ energy migration is limited. To optimize the efficiency in highly doped Mn4+ phosphors, a synthesis procedure aimed at reducing quenching sites (defects, impurity ions, Mn2+, and Mn3+) will be crucial.
[Senden T, van Dijk-Moes R J A, Meijerink A. Quenching of the red Mn4+ luminescence in Mn4+-doped fluoride LED phosphors[J]. Light: Science & Applications, 2018, 7(1): 1-13.]
另外在[Verstraete R, Sijbom H F, Joos J J, et al. Red Mn4+-doped fluoride phosphors: why purity matters[J]. ACS applied materials & interfaces, 2018, 10(22): 18845-18856.]中,Mn3+的存在会影响optical absorption behavior。
IVCT解释Ca2Al2SiO7: Eu2+/Eu3+发光的热稳定性
参考[Sci China Mater-2019],该基质的bandgap比较大,The large bandgap suggests a large energy barrier for the excited Eu2+: 5d electrons thermally ionized into CB. 另外该荧光粉的斯托克斯位移很小,this implies that, the energy barrier for energy level crossover relaxation between 4f and 5d is large。而two widely accepted mechanisms responsible for the thermal quenching route of Eu2+, namely, thermal ionization and thermally induced 4f and 5d level intersystem crossover relaxation. 但是这两种机理are hard to occur in present case, because it should overcome a large energy barrier as we analyzed above, yet a relative low temperature of 330 K cannot afford such energy. 所以用Jonas的IVCT理论解释,如下的位型坐标图。
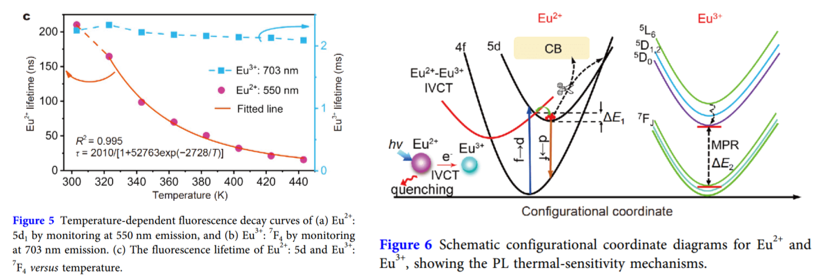
另外作者用多声子弛豫模型解释了Eu3+良好的热稳定性,即使考虑基质中最大的声子能量,也需要多达11个有效声子去弛豫。
IVCT Eu2+/Eu3+ 在CaF2、 SrF2 、BaF2的系统研究
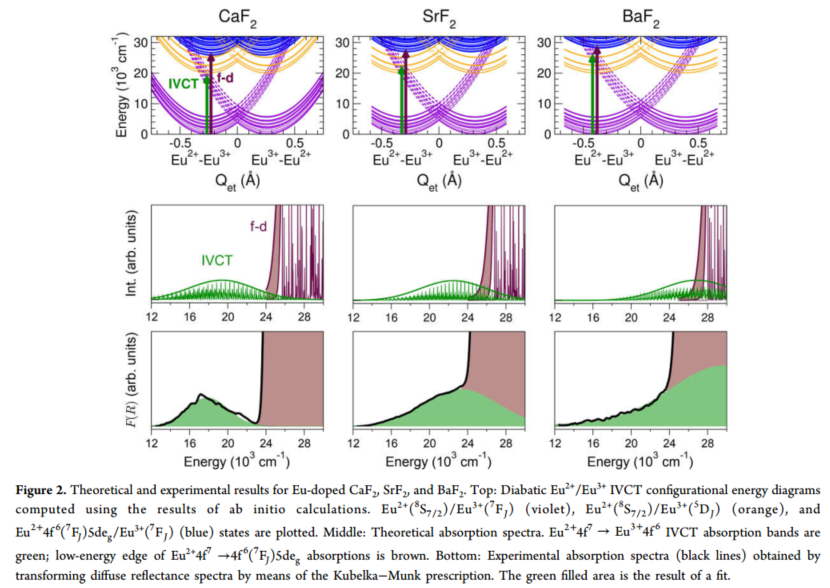
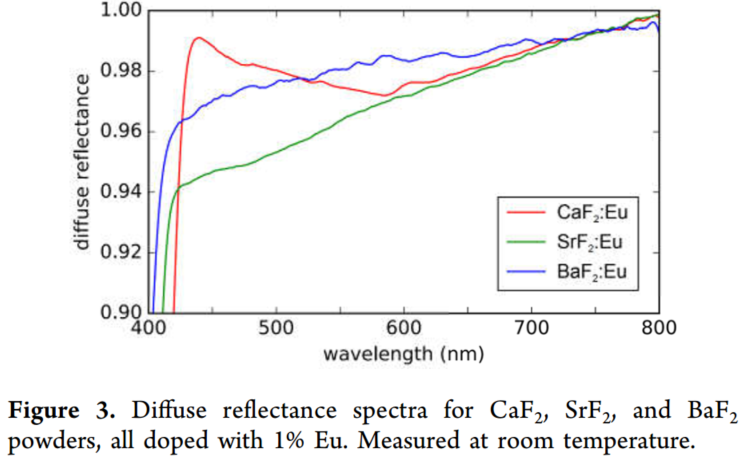
(1) 展示的结论:show direct evidence of IVCT states of Eu
(2) 实验和计算方法:
(a) diffuse reflectance spectroscopy
(b) state-of-the-art multiconfigurational ab initio calculations.
(3) 实验现象:
- a broad and flat absorption band is identified as an Eu2+ 4f → Eu3+ 4f IVCT transition.
- A host-driven energy shift of the IVCT absorption band to higher energies.
- IVCT and 4f−5d absorption bands are well separated in CaF2:Eu, get closer in SrF2:Eu, and overlap significantly in BaF2:Eu (case A, crossings provide a nonradiative decay channel to the relaxed mixed-valence ground state and an explanation to the fact that the blue Eu2+ 5d−4f emission observed in CaF2 and SrF2, is quenched in BaF2.).
(4) 计算和分析要点:
- we assumed that Eu3+ is nonlocally compensated, and, in consequence, the IVCT diagram is symmetric. 计算的IVCT图更准确的说法是electron-transfer reaction coordinate.
- A host-driven shift of the IVCT band toward higher energies is evident. This shift is caused by the increase in the mismatch between the Eu2+−F− and Eu3+−F− bond lengths across the CaF2−SrF2−BaF2 series. In effect, this mismatch is directly related to the horizontal offset between the two equivalent minima in the electron-transfer reaction coordinate \( \left[\Delta Q_{\mathrm{et}, \mathrm{e}} \approx 4\left(\mathrm{~d}_{\mathrm{Eu}^{2+}-\mathrm{F}^{-}}-\mathrm{d}_{\mathrm{Eu}^{3+}-\mathrm{F}^{-}}\right)\right]\), and the increase in \(\Delta Q_{\mathrm{et}, \mathrm{e}}\) from 0.512 (CaF2) to 0.632 (SrF2) to 0.782 Å (BaF2) produces an associated increase in the IVCT peak energy, which is only slightly compensated by the decrease in the EuF8 breathing mode vibrational frequencies across the series.
- Energy barriers for IVCT nonradiative decay show a clear trend from CaF2 to SrF2 to BaF2, 797, 374, and 95 cm−1, which is significant despite the diabatic approximation (非绝热近似); adiabatic avoided crossings could decrease their values but would keep their trend in the host series, and hence Eu2+ \(4 \mathrm{f}^{6}\left({ }^{7} \mathrm{~F}_{J}\right) 5 \mathrm{de}_{\mathrm{g}}\) luminescence quenching should be expected in BaF2.
注:我们在有关“Marcus电荷转移理论”的笔记讨论了非绝热和绝热两种情况。
(5) 反射率测试要点:
- 1 %的Eu掺杂浓度,this is a doping concentration that is representative of most applications, including LED phosphors, and that is still sufficiently low to avoid the formation of Eu aggregates.
- Because of the low absorption strength of these low-energy bands, diffuse reflectance spectroscopy requires a white baseline obtained from undoped fluorides.
- 所有的样品都underwent the same thermal treatment as the doped samples, ensuring that only absorption features due to the presence of Eu were measured.
(6) IVCT absorption band的拟合:The IVCT absorption band was fitted with a Voigt spectral profile,31 allowing a rough estimation of its position and its width.
(7) IVCT absorption band的intensity:Not a very stable value in these mixed-valence doped materials because it is a very complex property that depends on the actual distribution of Eu2+ and Eu3+ ions across the samples: It depends on the distribution of concentrations of Eu2+/Eu3+ pairs with different Eu2+−Eu3+ distances (because the oscillator strength is distance-dependent), which, in turn, depends on the doping concentration and exact synthesis conditions, although not in a straightforward fashion.
(8) 点评:the impact of the quenching activity of IVCT states on the phosphor performance can be substantial even when relatively few mixed-valence pairs are present. 这是因为即使在低掺杂浓度下,energy can be transferred between Eu2+ ions, on the one hand, and thermal IVCT makes the Eu2+/Eu3+ pairs distribution across the sample dynamic, on the other hand, altogether making the IVCT nonradiative decay path virtually accessible to all Eu2+ ions. Nonradiative IVCT decay can hence be a microscopic explanation for concentration quenching. As an example, a study of the blue phosphor BaMgAl10O17:Eu showed that the oxidation of only a small part of Eu2+ has a much more severe effect on the luminescence efficiency than one would expect from the decreasing Eu2+ content.
以上参考[Jonas-JPCL-2019]
CaS:Eu2+, Tb3+的MMCT近红外发光研究
xxxx
Eu2+到Ln3+的MMCT的系统性研究
这里来自[Jonas-JCP-2021],标题为Charge transfer from Eu2+ to trivalent lanthanide co-dopants: Systematic behavior across the series
电荷转移 for technological applications
(1) OSL for luminescence dosimetry or medical imaging
OSL是在electron backtransfer过程中出现的。IR or red light induces the OSL, having a sufficiently low photon energy to avoid the direct excitation of nonoxidized donors. If the OSL wavelength is shorter, a competition between nonoxidized donors and reduced acceptors (filled traps) can emerge, effectively limiting the performance of current state-of-the-art persistent phosphors.(Tydtgat的OME和David的Acs Photonics)
Many more technological applications rely on electron transfer processes among dopants or with other crystallographic defects. [Jonas-2021-PRB] 这里作者列出的几个参考文献,分别对应不同的应用。
(2) zero-thermal quenching phosphor
(3) SrAl2O4:Eu, Dy长余辉材料
(4) 自还原荧光粉,defects as useful reducing agents.
One of the most prominent candidates of luminescent defects are color centers (F centers), which are localized electrons in anion vacancies. 在卤化物中,人们对其光谱和magnetic resonance都有了很深入的研究,比如X射线荧光粉BaFBr:Eu2+和辐照之后的Ln3+掺杂的CaF2。[Underestimated Color Centers: Defects as Useful Reducing Agents in Lanthanide‐Activated Luminescent Materials- Angewandte-2020]
(5) 基于电荷转移的IR发光
(6) 能量存储(storage or long-afterglow phosphors),a trivalent co-dopant is typically added to the Eu2+ activator, introducing a metastable metal-to-metal charge transfer (MMCT) state. [Jonas-JCP-2021]
在硕士论文中,写到“值得注意的是,很多适合于Eu3+到Eu2+自还原的基质同时也是长余辉或者光能存储的材料,这可能是因为自还原现象和长余辉发光都与材料中的缺陷有关。”
电荷转移limit the performance
(1) blue Eu2+ 5d−4f emission observed in CaF2 and SrF2, is quenched in BaF2.
Photon controlled electron juggling between lanthanides in compounds
https://pubs.acs.org/doi/pdf/10.1021/acs.chemmater.0c02449
https://iopscience.iop.org/article/10.1149/2.0281609jss/pdf
https://www.sciencedirect.com/science/article/pii/S0022231322000308#!