3.YAG:Ce,Yb
[Photo-induced luminescence degradation in Ce, Yb co-doped yttrium aluminum garnet phosphors-Applied Optics-2018]
Reversible Valence Change of the Europium Ion Doped in Alkaline-earth Tetraborates
基本参数和辐照源
还原势(redox potential)
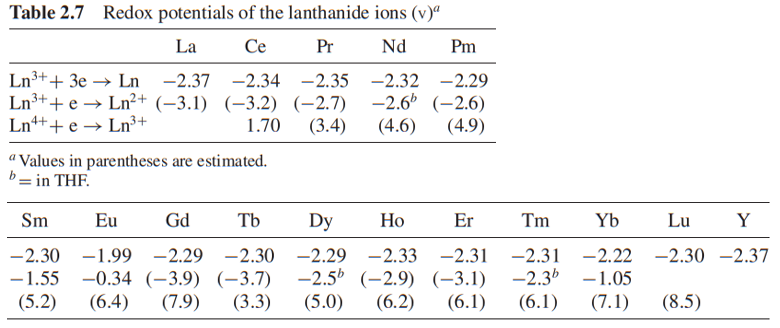
注:
(1) 上表来自Lanthanide and Actinide Chemistry, Wiley, Chichester, United Kingdom (2006), pp. 20-21
(2) 这里的THF应该是四氢呋喃Tetrahydrofuran。
(3) Eu这里的第二行的数值是-0.34,有的认为是0.35。
(4) 上表的单位是 eV。
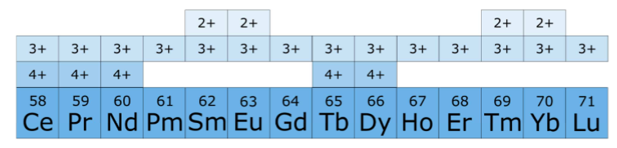
(5) 最下面的表说明了各种稀土常见的价态。

Rare earth elements have similar configurations in the two outermost shells. They exhibit typical metallic properties in chemical reactions. They tend to lose three electrons and exhibit a \(3+\) valence state. From the Periodic Table of the elements, rare earth elements are classed as less reactive than alkali metals and alkaline earth metals but more reactive than other metals. They should be stored in an inert liquid otherwise they will be oxidized and lose their metal luster. The metal reactivity increases gradually from scandium to lanthanum and decreases gradually from lanthanum to lutetium. That is to say, lanthanum is the most reactive metal of the 17 rare earth elements. Rare earth metals can react with water and release hydrogen. They react more vigorously with acids but do not react with bases.
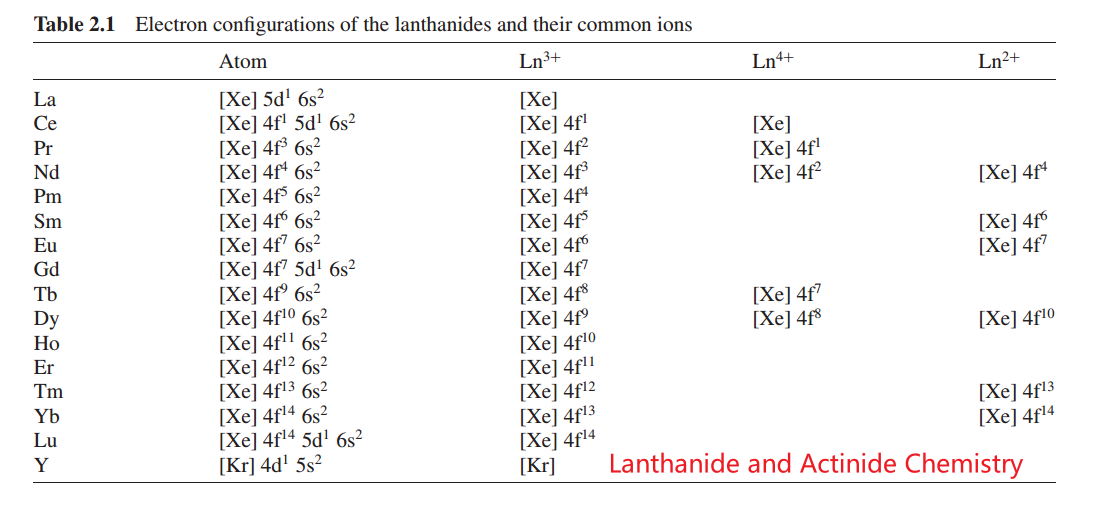
According to Hund's rule, electron shells are stable when empty, full or half-full. For example, the configurations \(4 \mathrm{f}^0\left(\mathrm{La}^{3+}\right), 4 \mathrm{f}^7\left(\mathrm{Gd}^{3+}\right)\), and \(4 \mathrm{f}^{14}\left(\mathrm{Lu}^{3+}\right)\) are stable. \(\mathrm{Ce}^{3+}, \operatorname{Pr}^{3+}\), and \(\mathrm{Tb}^{3+}\) have one or two more electrons than required for stable electronic configurations so they can be further oxidized to a \(4+\) state. In contrast, \(\mathrm{Sm}^{3+}, \mathrm{Eu}^{3+}\), and \(\mathrm{Yb}^{3+}\) have one or two less electrons than required for a stable electronic configuration and they, therefore, tend to receive one or two electrons and undergo a reduction to a \(2+\) state. These are the reasons for these elements having abnormal valence states.
Standard reduction potentials, \(E_{\mathrm{Ln}^{4+}}^{\circ} / \mathrm{Ln}^{3+}\) and \(E_{\mathrm{Ln}^{3+}}^{\circ} / \mathrm{Ln}^{2+}\), represent the driving force stability of the reduction state. The more positive the value of \(E_{\mathrm{red}}^{\circ}\) the greater the driving force for reduction. The standard reduction potentials of rare earths are shown in Table \(1.3\).
The data shown in the table indicate that when comparing \(E_{\mathrm{Ce}^{4+}}^{\circ} / \mathrm{Ce}^{3+}\) with \(E_{\mathrm{Tb}^{4+}}^{\circ} / \mathrm{Tb}^{3+}\) electronic pairs, to act as an oxidizing agent, \(\mathrm{Tb}^{4+}\) is stronger than \(\mathrm{Ce}^{4+}\); to act as a reducing agent \(\mathrm{Ce}^{3+}\) is stronger than \(\mathrm{Tb}^{3+}\). When comparing \(E_{\mathrm{Eu}^{3+} / \mathrm{Eu}^{2+}}^{\circ}\) with \(E_{\mathrm{Yb}^{3+} / \mathrm{Yb}^{2+}}^{\circ}\), to act an oxidizing agent \(\mathrm{Yb}^{2+}\) is stronger than \(\mathrm{Eu}^{2+}\) under the standard conditions. Figure \(1.11\) visualizes this trend. The transverse axis is the atomic number and the length of the short lines along the vertical axis represents the trend of valence state variation. [Rare earth coordination chemistry: fundamentals and applications]
电离能(Ionization Energies)
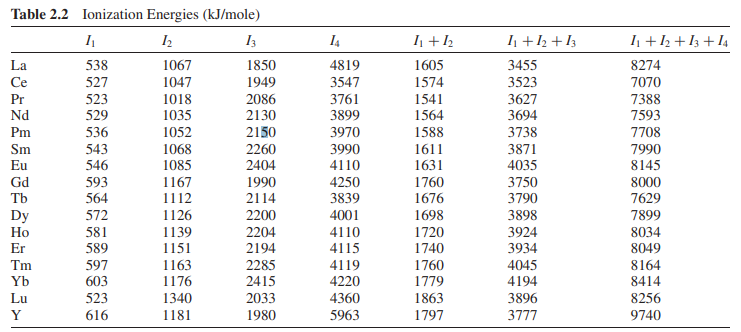
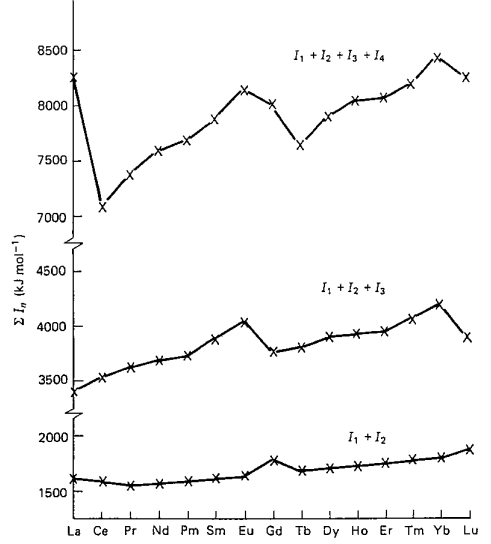
注:
(1) 上图也是来自Lanthanide and Actinide Chemistry
(2) 电离势(ionization potential)和电离能是同一个意思,但是前者是an older and obsolete term。
电离辐射是指携带足以使物质原子或分子中的电子成为自由态,从而使这些原子或分子发生电离现象的能量的辐射
待续。。。。
价态调控及其应用
多种价态共存-概述
For multivalent dopants (e.g. Ce, Eu or Mn) a reduction is also often required. For cerium this step clearly aims at stabilizing the luminescent Ce3+ instead of the commonly non-luminescent Ce4+. Even though, the possible coexistence of the two oxidations states of the dopant is not much considered, and a single oxidation state of the activator only is often expected to be stabilized. However, even under severe reducing conditions, a mixed valence may exist.
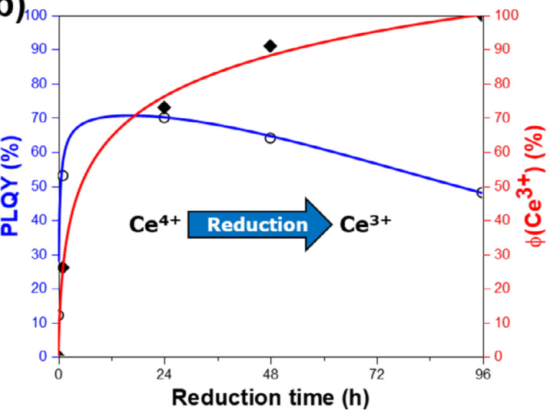
- YAG:Ce即使在强还原气氛下处理,XPS测试也显示出Ce4+的存在;
- CaSc2O4:Ce,slight Ce3+ concentration modifications导致drastic luminescence efficiency changes.
- Strong reductions might not always be ideal to reach the best efficiencies.
- Since reduction leads to a homogeneous distribution of the dopants with different oxidation states in the materials, these results are of great importance for the control of a high diversity of tunable properties such as luminescence, absorption, conductivity or magnetism. The better understanding of the dopants reduction mechanism and its control will help improving a large variety of applications, among them: LEDs, sensors, transparent semi-conductors, bio-imaging.
以上来自[Applied Materials Today-2020]
自还原理论
我的描述:In general, to obtain Eu2+-activated luminescent materials, a reduction-atmosphere of H2, H2/N2, or CO is always required because of the necessary reduction process of Eu3+ → Eu2+. However, in some unique compounds, like phosphate Ba3(PO4)2, borate SrB4O7, silicate BaMgSiO4 and aluminate Sr4Al14O25, it was found that the self-reduction of Eu3+ to Eu2+ could occur in an air or O2 atmosphere at high temperatures. This anomalous self-reduction phenomenon can be partially ascribed to the rigid three-dimensional (3D) structures, with tetrahedral anion groups such as BO4, SiO4, AlO4, or PO4.
Four conditions that seem to be necessary for the reduction Eu3+→ Eu2+ in solid state compounds when prepared in air at high temperature.
- no oxidizing ions are present in the host compounds;
- the dopant trivalent Eu3+ ion replaces a divalent cation in the host;
- the substituted cation has a similar radius to the divalent Eu2+ ion;
- the host compound has an appropriate structure, based upon tetrahedral anion groups (BO4, SO4, PO4 or AlO4).
以上是来自传统的自还原理论,下面详细记录一下Markus Suta的文章。
- Inorganic hosts, such as SrB4O7 or certain nitrides, intrinsically stabilize Eu2+ even when the dopant is an Eu3+-based precursor and reducing conditions are not employed in the synthesis. 作者展示了trapped charge carriers, such as color centers (localized at defects), can also act as redox partners to stabilize certain oxidation states of activators.
- Chemically, the stabilization of divalent lanthanides in inorganic host compounds is still a challenging task for most of these species and limits potential application areas. Eu3+的还原最容易,也最常见(还原化学势可以看出来)。对于Eu2+-based或者Eu2+-activated solid compounds来说,想要从Eu3+-based precursors得到Eu2+,需要
-
- 将原料还原:with H2 gas at elevated temperatures (> 400 oC), but also with LiH or liquefied NH3.
- 特殊host (oxidizable soft anions):containing polarizable anions with appreciable covalent bonding character, such as iodides, hydrides, heavy chalcogenides, and nitrides, readily stabilize Eu2+, sometimes even upon use of Eu3+-based starting materials.
原因:The stability of Eu2+ in the previously mentioned classes of compounds is chemically intuitively evident by means of Pearson's hard and soft acid and base (HSAB) principle. Moreover, these anions are generally oxidizable and it is thus also mechanistically clear how Eu3+ ions can be readily reduced in those compounds even without explicit reducing conditions during the synthesis at high temperatures as elemental by-products stemming from the anions can also be formed then.
- SrB4O7:没有oxidizable soft anions,the mechanism of the inherent reduction is still under debate. It was speculated that the intrinsic reducing action of SrB4O7 stems from potential charge-compensating defects present in SrB4O7 that allow reduction of the trivalent activators. Alternatively, a potential impact of the rigid network built up by the composing [BO4]5- units was also anticipated, and it could be shown that this is clearly related to a long-range ordered crystalline structure of the host material.
-
- Defects的多面性:
-
- 猝灭发光:Mostly, defects are considered undesirable in phosphors as they can act as trapping sites for charge carriers and thereby diminish the luminescence efficiency or completely quench it.
- 长余辉:In fact, this particular, apparently undesirable feature of defects has found a useful application in the field of persistent phosphors.
- 稳定价态:However, a role as redox-active centers to help stabilize luminescent dopants in a desirable oxidation state could prove to be a beneficial alternative concept for a simple chemical access to new phosphors.
-
- STEs: are commonly encountered in both halidoperovskites and other halides, especially if the band gap is successively lowered and they obtain more semiconducting character.
- CsMgX3基质的特点:
-
- 特殊的晶体结构feature strongly favors polaron formation and self-trapping of excitons within these chains.
- Eu2+和Mg2+的半径不匹配 favors exciton localization at the Eu2+ impurities based on the very strong induced distortion as was also demonstrated by us earlier.
-
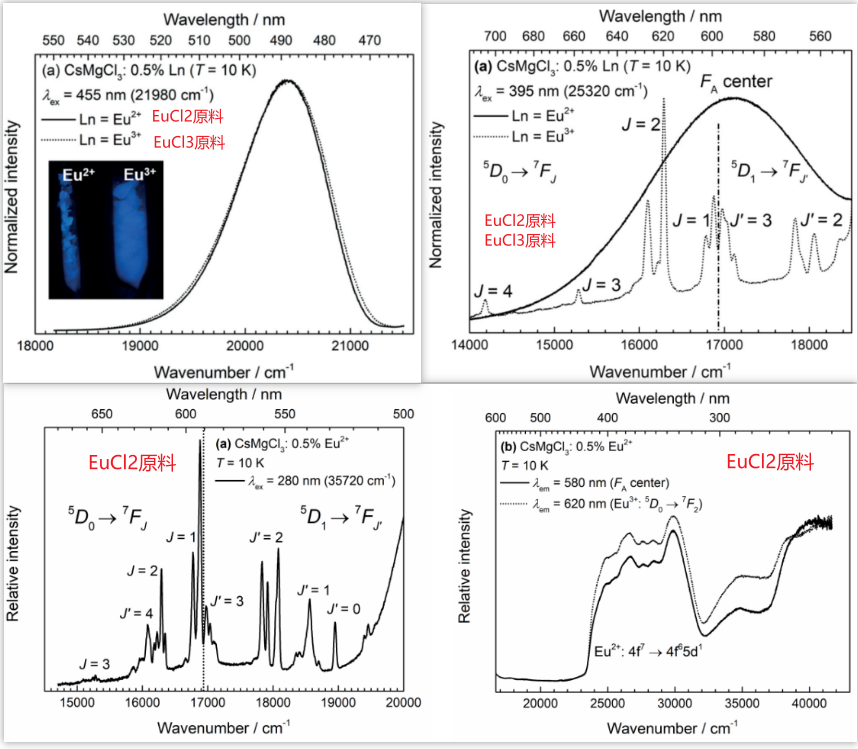
- 标号上面两个分别为(a)和(b),下面两个分别为(c)和(d);
- 图(b)中的红色宽带发光只有在低温下才能观察到,室温下完全猝灭,作者认为这个发光峰对应于 F centers,更确切地说是activator localized FA center,因为:
-
- 峰位置、宽度都和文献报道的F centers的发光类似;
- UV激发,观察到Eu3+的发光,图(c)。
-
- 图(d),检测Eu3+的发光,得到的激发光谱是和Eu2+的激发谱类似。
Thus, the observed luminescence of Eu3+ is intrinsically related to the presence of Eu2+ ions, which is already a strong indication for the anticipated charge carrier trapping mechanism explained above. - 测试了FA center的寿命为0.1 ms, which is expected for defects。
- 采用EuCl3作为原料,no reductive conditions during synthesis yet leads to an observation of strong Eu2+.
- 为了排除a potential thermally induced reduction of EuCl3 during synthesis,采用了Eu2O3,同样得到了Eu2+的发光。
- Eu3+作为 potential electron scavengers,之前Eu2+作为原料观察到的 broad red emission band在这里vanishes,而部分没有被还原的Eu3+的发光现在可以观察到了。
- the Eu3+-IL-STE entity now more resembles a “Eu2+ + h+” Frenkel-type exciton. (紧束缚激子或者叫弗仑克尔(Frenkel)激子,它的束缚半径比较小,大约在一个原子的范围内。激子是一种localized,想要变为delocalized需要吸收足够的激子结合能。)
对于之前EuCl2作原料的情况,作者说Eu2+-IL-STE entity can be chemically envisioned as a “Eu3+ + e-” Frenkel-type exciton. - 文章最后也提到了,Strong evidence for the presence of trapped electrons was given by combined thermoluminescence and electron paramagnetic resonance studies in the borate Zn4B6O13. 在A Meijerink的这篇文章中,作者通过测试UV辐照后的样品的EPR表面electrons trapped in two types of oxygen vacancies, electrons trapped on or near Mn2+ impurities and holes trapped by borate groups.
参考文献:
(1) Z. W. Pei, Q. Su and J. Y. Zhang, J. Alloys Compd., 1993, 198, 51.
(2) M. Y. Peng, Z. W. Pei, G. Y. Hong and Q. Su, J. Mater. Chem., 2003, 13, 1202
(3) Underestimated Color Centers: Defects as Useful Reducing Agents in Lanthanide-Activated Luminescent Materials-Angew-2020
原料(或者加还原剂)和气氛(热处理工艺)调控价态
(1) Eu原料
stabilization of Eu2+ could be improved when the doping was executed using EuF2 or EuS in the reacting mixture of oxides. 这句话来自[JAC-Eugeniusz Zych-2020]
比如[M.Nikl-JPCC-2016]用的就是EuS、EuF2。[Underestimated Color Centers: Defects as Useful Reducing Agents in Lanthanide-Activated Luminescent Materials-Angew-2020] 也用了EuCl2。
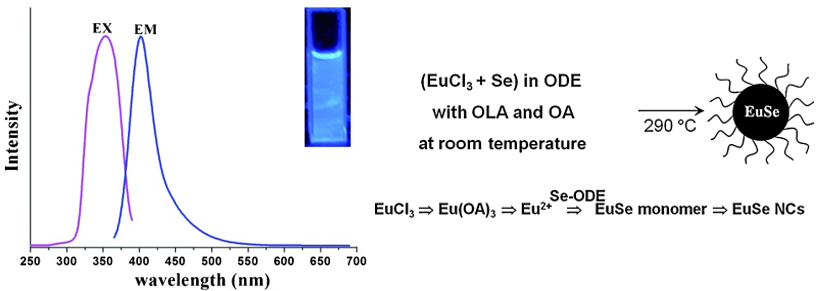
(2) 湿化学合成中的还原剂
amine作为reducing agent制备了EuSe nanocrystals,观察到了Eu2+的发光[A Simple Reducing Approach Using Amine To Give Dual Functional EuSe Nanocrystals and Morphological Tuning-Angew-2011]。
(3) 气氛-CO
By adding fluxes and sintering in a CO reducing atmosphere, the IQE is greatly enhanced to 92.3%. 之前别人报道的相同基质掺杂Cr3+效率不高是因为Cr3+ luminescence suffered from impurities and oxidation of Cr4+ when the material was sintered in air.
[Strategies to approach high performance in Cr3+-doped phosphors for high-power NIR-LED light sources]
之前研究的固相法烧制的Cr3+多集中在含Ga/Ge的基质,这些元素在还原性气氛下容易挥发,不能形成纯相。
我:注意气氛既可以是烧制过程中用还原性气氛,也可以是air中烧制好后,在还原气氛下post-treatment。
(4) 各种气氛-概述
One valence state of dopant can be preferentially stabilized at the expense of the other according to the partial pressure of oxygen. Thus a highly oxidative atmosphere such as O2 or air, will favor the stabilization of the oxidized form of dopant. On the contrary, with the use of a reductive atmosphere such as CO or H2, the reduced form of dopant will be predominantly stabilized. This has been demonstrated for example through the study of cerium-doped materials.
 Thus, under oxidative atmosphere (in air), oxidation of Ce3+ ions into Ce4+ is favored, which leads to the decrease of the luminescence intensity. In reductive atmosphere (CO), the ratio Ce3+/Ce4+ increases, improving the luminescence intensity. In N2 atmosphere,the material is isolated from any oxidative or reducing sources. Thus, an intermediate emission intensity corresponding to a limited stabilization of Ce3+ ions is observed.The charge balance is preserved owing to the formation (respectively filling) of oxygen vacancies in reducing (respectively oxidative) atmosphere. The study of Ce4+/Ce3+ doped materialsillustra tes the possibility of tuning the ratio of the dopant valence state according to synthesis conditions, which can be generalized to most of thedopants.
Thus, under oxidative atmosphere (in air), oxidation of Ce3+ ions into Ce4+ is favored, which leads to the decrease of the luminescence intensity. In reductive atmosphere (CO), the ratio Ce3+/Ce4+ increases, improving the luminescence intensity. In N2 atmosphere,the material is isolated from any oxidative or reducing sources. Thus, an intermediate emission intensity corresponding to a limited stabilization of Ce3+ ions is observed.The charge balance is preserved owing to the formation (respectively filling) of oxygen vacancies in reducing (respectively oxidative) atmosphere. The study of Ce4+/Ce3+ doped materialsillustra tes the possibility of tuning the ratio of the dopant valence state according to synthesis conditions, which can be generalized to most of thedopants.
[Tuning the oxidation states of dopants: a strategy for the modulation of material photoluminescence properties-Chemistry–A European Journal, 2021]
(5) oxygen getter(hydrides)
 Controlled reduction of dopant can also be performed using oxygen getter such as hydrides (e.g. CaH2, NaH, LiH) as reducing agent. This method can be implemented after material synthesis if the dopants are the only reducible elements in the material. It consists in the thermally activated disinsertion of oxygen from the crystal structure of the phosphor. Disinserted oxygen can then react with the getter to form thermodynamically stable oxides. Consequently, the dopants are reduced to keep the charge balance. Since this process is temperature and time dependent, it is possible to accurately control the valence states of dopants as it has been demonstrated by Behrh etal. through the investigation of SrAl2O4:Eu phosphor. The reduction of Eu3+ ions into Eu2+ is performed with CaH2 as oxygen getter. With the increase of reduction temperature, the Eu2+ emission intensity increases at the expense of Eu3+ emission intensity.
Controlled reduction of dopant can also be performed using oxygen getter such as hydrides (e.g. CaH2, NaH, LiH) as reducing agent. This method can be implemented after material synthesis if the dopants are the only reducible elements in the material. It consists in the thermally activated disinsertion of oxygen from the crystal structure of the phosphor. Disinserted oxygen can then react with the getter to form thermodynamically stable oxides. Consequently, the dopants are reduced to keep the charge balance. Since this process is temperature and time dependent, it is possible to accurately control the valence states of dopants as it has been demonstrated by Behrh etal. through the investigation of SrAl2O4:Eu phosphor. The reduction of Eu3+ ions into Eu2+ is performed with CaH2 as oxygen getter. With the increase of reduction temperature, the Eu2+ emission intensity increases at the expense of Eu3+ emission intensity.
[A Chemical Route Towards Single-Phase Materials with Controllable Photoluminescence-Angew-2015]
[Tuning the oxidation states of dopants: a strategy for the modulation of material photoluminescence properties-Chemistry–A European Journal, 2021]
Electron beam(处理)和laser beam(合成/处理)
electron beam参考菲利普的ome,
laser beam: Comparison among the spectra revealed the presence of the single trivalent state of Tb in the YAG laser-sintered ceramics. These results agree with those previously reported that suggested laser sintering in air can create a reducing environment at high temperature and this causes the release of oxygen, creating oxygen vacancies inside the sample. This oxygen deficiency associated with the high cooling rate promote the stabilization of the lower chemical state of rare earthss, including Eu2+ and Ce3+, and transition metals, Ti3+, even when the samples were sintered in air. Therefore, during the sample cooling down, the re-oxidation is frustrated and just the Tb3+ became stable at room temperature. [Laser sintering and photoluminescence study of Tb-doped yttrium aluminum garnet ceramics]
共掺杂调控价态(电荷补偿)
概述:In order to prevent defect formation, charge compensators can also be inserted inside the material. This strategy is widely investigated for preferential stabilization of one oxidationstate of dopant, leading to the enhancement of luminescence properties. Li+, Na+, and K+ are among the most widely used monovalent charge compensators but other multivalent charge compensators such as Mg2+, Si4+ or Ge4+ ions have alsobeen investigated.
[Tuning the oxidation states of dopants: a strategy for the modulation of material photoluminescence properties-Chemistry–A European Journal, 2021]
(1) 共掺杂Mg2+/Ca2+
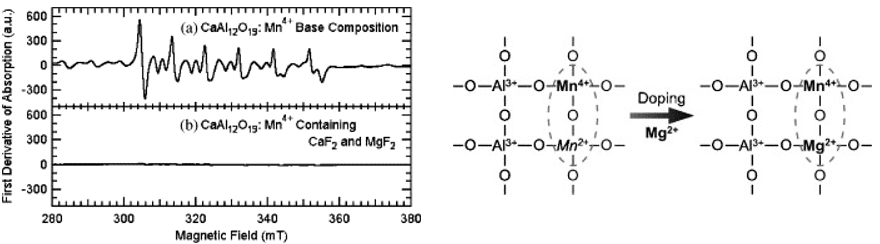
T. Murata等在2005年在CaAl12O19:Mn4+中通过添加MgF2或者CaF2来促进Mn4+的发光[JL-2005],减少样品中Mn2+的出现。如图是CaAl12O19:Mn4+和加入了 CaF2/MgF2的CaAl12O19:Mn4+的EPR谱,虽然作者没有发现Mn2+的发光,但是在 CaAl12O19:Mn4+样品中仍显示出明显的Mn2+的信号,而加入CaF2/MgF2之后, Mn2+的信号消失,以Mg2+为例,作者把原因归结为Mn4+-O-Mn2+这种电荷补偿形式被Mn4+-O-Mg2+所取代。MgF2不仅可以作为助熔剂,促进高温固相反应的进行,也作为电荷补偿剂,抑制了Mn2+,因而最终Mn4+的发光得到了显著增强。
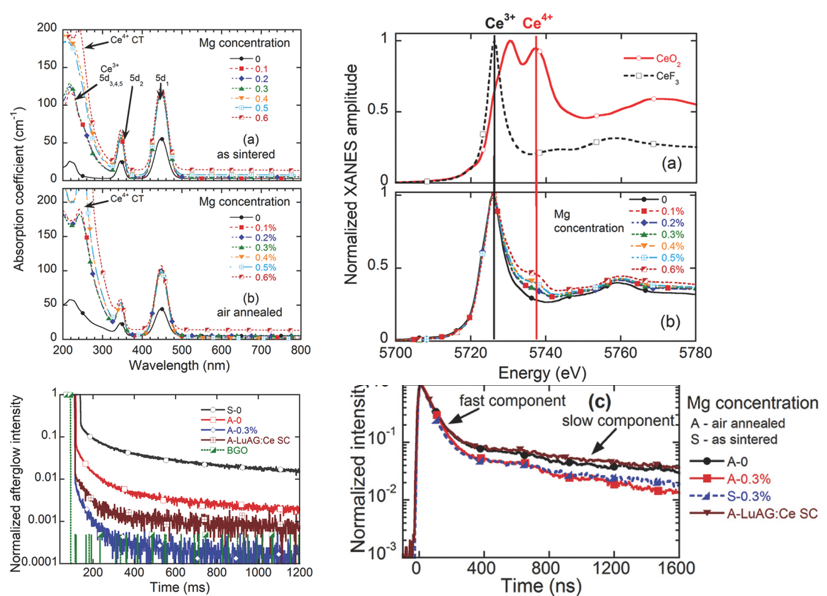 Lu3Al5O12:Ce,Mg Optical Ceramics Scintillators [AOM-2016]通过共掺杂Mg2+,促进了Ce3+部分地转变为Ce4+。The Ce4+ center, however, does not impair the scintillation performance due to its capability to positively influence the scintillation process. The importance of simultaneous application of such co-doping and annealing treatment is also demonstrated. With 0.3 at% Mg, our ceramics display a light yield of ≈25000 photons/MeV with short 1 μs shaping time, a relative fast component intensity as high as 60%, and very low afterglow.
Lu3Al5O12:Ce,Mg Optical Ceramics Scintillators [AOM-2016]通过共掺杂Mg2+,促进了Ce3+部分地转变为Ce4+。The Ce4+ center, however, does not impair the scintillation performance due to its capability to positively influence the scintillation process. The importance of simultaneous application of such co-doping and annealing treatment is also demonstrated. With 0.3 at% Mg, our ceramics display a light yield of ≈25000 photons/MeV with short 1 μs shaping time, a relative fast component intensity as high as 60%, and very low afterglow.
In Ce3+-doped garnet scintillators, Ce4+ is intentionally added to accelerate the decay and, hence, improve the time response of the radiation detector. [Charge transfer from Eu2+ to trivalent lanthanide co-dopants: Systematic behavior across the series-JCP-2021]
Role of Ce4+ in the Scintillation Mechanism of Codoped Gd3Ga3Al2O12∶Ce
The Carrier Capture and Recombination Processes in Ln3+-Activated Scintillators
(2) 共掺杂Ln3+/Bi3+
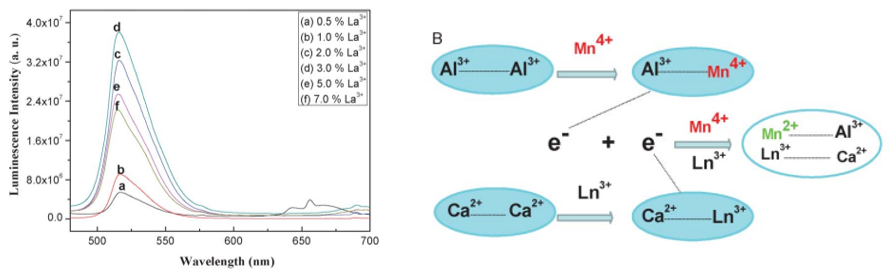 潘跃晓等人研究了在 CaAl12O19中[RSC-2013],通过共掺杂三价离子(Bi3+, La3+等) ,在空气气氛下实现 Mn4+→Mn2+空气气氛下的自还原。
潘跃晓等人研究了在 CaAl12O19中[RSC-2013],通过共掺杂三价离子(Bi3+, La3+等) ,在空气气氛下实现 Mn4+→Mn2+空气气氛下的自还原。
(3) 共掺杂Hf4+
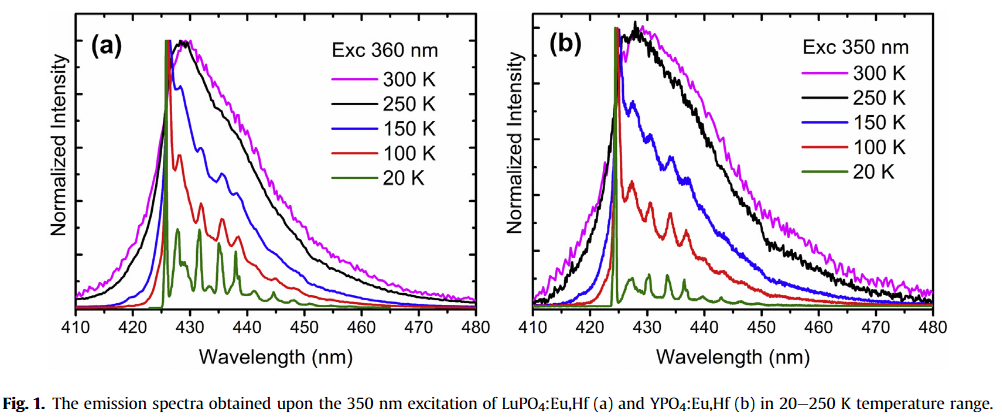 Stable Eu2+ ions in LuPO4 and YPO4 single crystals were obtained by co-doping with Hf4+. [JAC-2020]
Stable Eu2+ ions in LuPO4 and YPO4 single crystals were obtained by co-doping with Hf4+. [JAC-2020]
Electron transfer between Eu and Tb in complex fluorides
(4) 共掺杂Li+
补充
费米能级和稀土价态-Dorenbos
Dorenbos finally indicated that the redox stability of a given oxidation state of a lanthanide ion in an inorganic host should depend on the relative position between the ground state of the incorporated activator within the band gap and the Fermi level of the inorganic host material. [Underestimated Color Centers: Defects as Useful Reducing Agents in Lanthanide-Activated Luminescent Materials-Angew-2020]
$$E_{\mathrm{Ff}} \equiv E^{\mathrm{CT}}-0.54 E^{\mathrm{ex}}$$这里的0.54来源于1.08的一半,\(E^{\mathrm{ex}}\)表示的是the energy needed to excite the host lattice (i.e. the energy to create a bound electron-hole pair (exciton))。
2+基态的能级高于费米能级(\(E_{\mathrm{Ff}}>0\)),那么Eu3+更稳定,如果Eu2+的能级低于费米能级,那么Eu2+更稳定。按照Dorenbos的说法,还原性气氛使得费米能级上移,氧化氢气氛使得费米能级下移,他还讨论了电荷补偿剂对Eu能级的影响(局域电荷补偿,不影响费米能级的位置,但是会影响Ln2+和Ln3+基态的位置。)。可以参考下图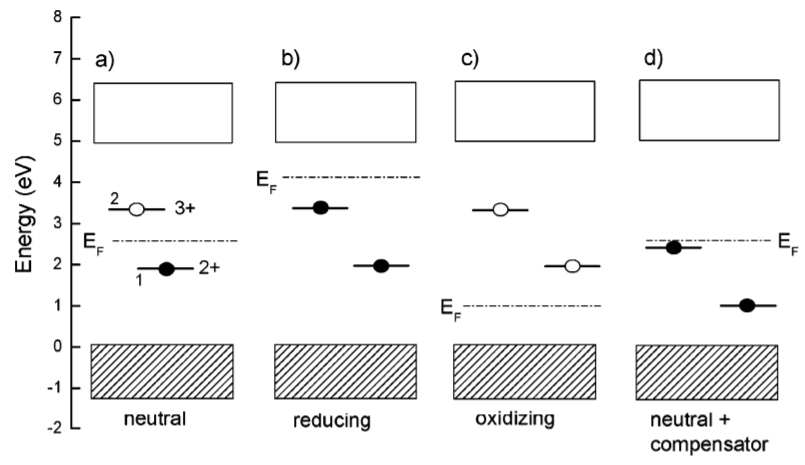
规律总结:
- 对氧化物来说,\(E_{\mathrm{Ff}}\)都是大于零,也就是说倾向于形成Ln3+;
- Ln半径越大,\(E_{\mathrm{Ff}}\)越小;
- bandgap越大,\(E_{\mathrm{Ff}}\)越小;
- Indeed there are very few reports about Eu2+ on trivalent rare earth sites in oxide compounds even when prepared under reducing conditions. (这是因为Eu2+的激发态往往很靠近导带或者直接在导带里面,导致发光不强或者猝灭)
- 对于硼酸盐来说,the rigid tetrahedron structure of anions prevents the attack of oxygen from air on Eu during synthesis. This is also the case for tetrahedral phosphate, silicate and aluminate group;
- SrB4O7这个物质值得特别注意,在这种基质里面,除了可以稳定Eu2+,也可以稳定Sm2+。它的禁带宽度在氧化物里面来说很大(10 eV)。

- \(E_{\mathrm{Ff}}\)比较大时,比如CaO (1.2 eV)、SrO (1.5 eV),即使还原气氛但是还是不能被完全还原。
3+/Eu2+能级和Eu2+/Eu3+
应用:
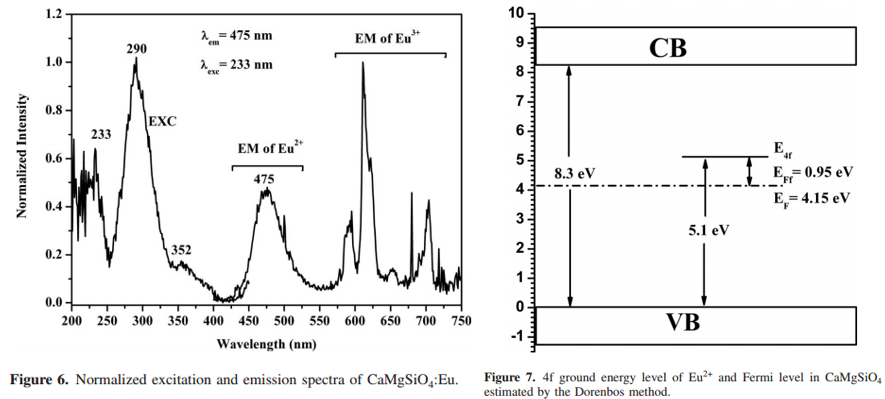
Eu2+掺杂的样品发光很弱。The energy difference between the first 5d state of Eu2+ and the bottom of the CB is 0.1 eV, which is consistent with the weak luminescence of Eu2+ in CaMgSiO4 and an incomplete reduction in Eu3+ in it. The energy difference (EFf) between the 4f ground level of Eu2+ and the Fermi level for CaMgSiO4 is calculated to be 0.95 eV, and this high EFf value may account for the incomplete reduction in Eu2+ in this compound. [Photoluminescence Properties and Valence Stability of Eu in CaMgSiO4-JES-2010]
Degradation和价态变化
(1) [Thermal degradation of the green-emitting SrSi2O2N2:Eu2+ phosphor for solid state lighting-JMCC-2014]
the thermal degradation of SrSi2O2N2:Eu2+ can be ascribed to (i) oxidation of activators (Eu2+ → Eu3+) and (ii) oxidation of the host crystal (SrSi2O2N2 → SrSiO3).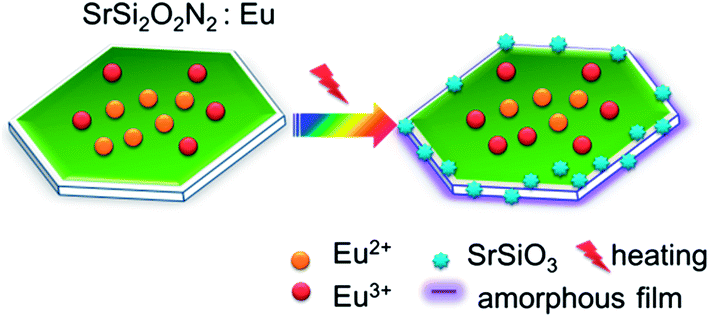
压力导致的价态变化
YAG中Yb2+和Yb3+的动态变化
Cr3+/Cr4+的调控
[Controlling Cr3+/Cr4+ concentration in single-phase host towards tailored super-broad NIR luminescence for multifunctional applications]能带工程调控Charge Transfer
不同价态离子含量的Quantification
It is important to note that it is not possible to precisely quantify the ratio of two oxidation states of one dopant using the photoluminescence properties only. Thus, the efficiencies of excitation and emission of activators depend strongly on many parameters including the excitation wavelength (see associated section), or absorption cross-section. Energy and charge transfers can also occur between ions, as well as non-radiative de-excitations (e.g. multiphonon relaxations via lattice vibration), leading to the variation of luminescence properties of emitting centers. Thus, in order to identify and quantify different oxidation states of dopant, several methods can be considered among which Electron Paramagnetic Resonance (EPR), Xray Photoelectron Spectroscopy (XPS), or X-ray Absorption Near Edge Spectroscopy (XANES).
[Tuning the oxidation states of dopants: a strategy for the modulation of material photoluminescence properties-Chemistry–A European Journal, 2021]
