一般性概述
(1) 两种类型的跃迁
- 对\(4f\)-\(4f\)稀土离子跃迁,weak coupling, \(S\approx 0\),因此吸收和发射的maxima都对应着ZPL的transitions。在这个过程中Ln3+-ligand的键不会有大的变化,这是intra-configurational transitions。受晶格的影响小(与晶格的耦合强度小),跃迁几率小,这就使得离子在激发态停留时间延长,从而容易将激发能量传给另一个离子,并可能不断地传下去,直到被异种离子或晶格缺陷所吸收,造成猝灭。
- 对\(4f\)-\(4f5d\)跃迁,bond(distance)会变,with intermediate electron-phonon coupling,\( S\)大。这是inter-configurational transitions.
(2) 基质对f-f emitter发光的影响:
- relative stength of the emission lines (via selection rules associated with local symmetry)
- splitting of the emission lines ( crystal field)
- QE (non-radiative pathway and therma quenching)
(3) 2+和Ce3+
- 2+ excitation spectrum is somewhat broader and flatter than the one for Ce3+, due to the large splitting of the 4f65d multiplet in Eu2+. At higher temperature, the absorption strength of Ce3+ somewhat reduces, due to a thermal population of the higher Stark levels of the 4f(2F5/2
- 2+的4f65d是一个宽劈裂,featureless [Philippe_JL_2012],而Ce3+的5d能级劈裂比较小,可以清晰区分出5d1和5d2
(4) MarkusSuta [Synthesis, spectroscopic properties and applications of divalent lanthanides apart from Eu2+-JL-2019]
(5) 允许跃迁的分类:[固体发光材料—P75]
- 易被氧化的稀土离子容易发生4f-5d跃迁:
- Sm2+/Eu2+/Yb2+
- Ce3+/Pr3+/Tb3+
- 易被还原的稀土离子容易发生电荷迁移跃迁:
- Sm3+/Eu3+/Yb3+
- Ce4+/Pr4+/Tb4+
- Nd3+/Dy3+/Ho3+/Er3+/Tm3+ (在电负性较小的硫化物中,吸收峰位于30000 cm-1附近)
(6) 屏蔽效应:Ln3+电子组态能级图中的能级实际上已经被晶体场分裂,但是由于5s2和5p6电子屏蔽的缘故,这种分裂作用非常小,以过渡金属离子dn为比较对象,当过渡金属离子所感受到的晶体场强度比Ln3+所感受到的晶体场强度高三个数量级。
(7) 无机稀土发光的几个milestones:(参考CSR-Bünzli-2009)
- Y2O3:Eu3+高效红色荧光粉,用于CRT和fluorescent lamp-1906;
- YAG:Nd3+激光-1964
- Er-doped optical fibres for telecommunications-1987
- 在氮化硅高温结构陶瓷中加入氧化钇等稀土,可大大改进它的高温性能,制成汽车用的电热塞,以代替金属的电热塞,延长使用寿命;
- 氧气传感器,以Y2O3稳定的ZrO2制成管状作为固体电解质,用铂作为电极,其基本原理是利用氧的浓差电池。利用此浓差电池所产生的平衡电动势自动调节和控制空气的流量,从而达到控制空气和燃料比的目的。
- 纯氧化镧是无色的,它是制备高级玻璃的重要原料,用它可制造出高折射率、低色散的镧玻璃。例如,使用镧玻璃可制造高级照相机、摄像机、航空摄影镜头、潜望镜、望远镜、长焦距镜头、变焦距镜头等光学仪器的光学元件,可获得高清晰度的图像。
- 核爆炸护目镜,在锆钛酸铅中加入镧制成的透明铁电陶瓷PZLT,可作为光开关,用于核爆炸时的护目镜。一旦核爆炸出现强光时,这种护目镜会立刻自动变色(可能只需要不到150微秒),把强光遮挡住而保护眼睛。
- La是稀土中第一个被观察到有超导现象的是金属镧,它的临界温度仍然很低,六方结 构的金属镧的Tc为3.9K,面心结构的Tc为5.4K。诺奖超导材料,镧钡铜氧La1-xBaxCuO4它是层状的复合氧化物。
- 储氢合金LaNi5H6,参考LaNi5H6 and Similar Alloys for Hydrogen Storage,Chem. Commun., 2021, 57, 9374-9377,B站视频
- 稀土Y型沸石催化剂,石油裂化的催化剂,其中的稀土是价格相对便宜的La和Ce。
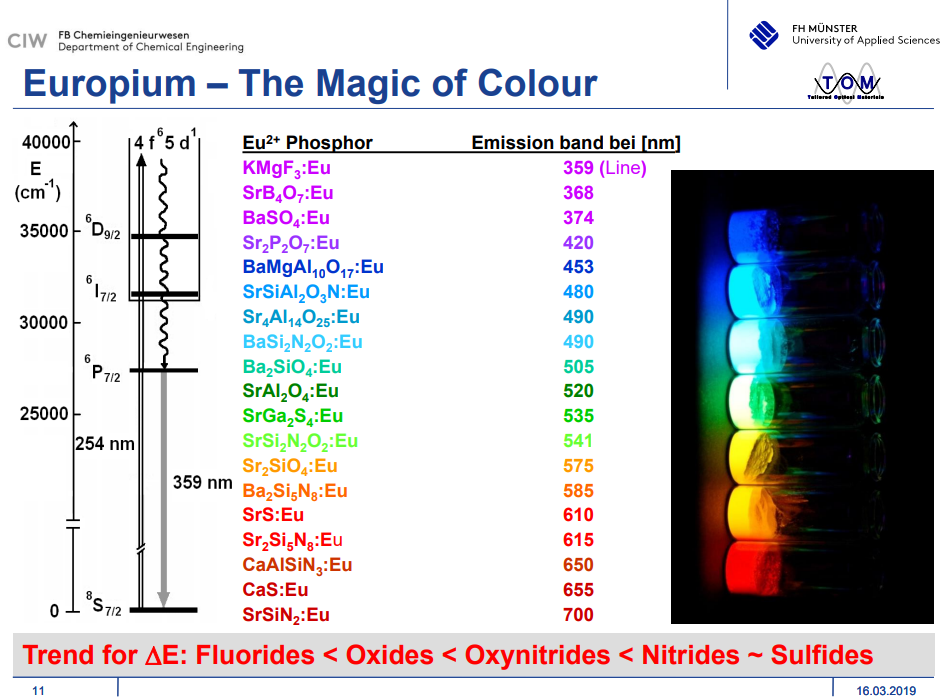
发射波长:
- Ce3+ may range from the ultraviolet region in fluorides like that of KMgF3 to the red region in sulfides like that of Lu2S3. (Phosphor Handbook)
- Ce3+的两个峰的强度和浓度的关系,以SrS:Ce3+为例,短波长发射峰对应\( 4f5d\rightarrow ^2F_{5/2}\),长波长发射峰对应\(4f5d\rightarrow ^2F_{7/2}\)。Because of shielding by the outer 5s and 5p electrons, the energy level structure for the 4f ground state of Ce3+ is fairly insensitive to host and crystal field effects and is depicted as two levels separated by ~2000 cm-1(spin–orbit coupling作用的结果).
- 在掺杂浓度不大时,变浓度对晶体场的影响不会对两个峰的强度比产生较大的影响。但是在掺杂浓度较大的时候,容易出现短波长峰被重吸收,看起来短波长峰相对于长波长的强度下降。3+
寿命:
- 5d 态电子寿命非常短,一般为 40~100 ns。The fast 15–60 ns 5d–4f emission of Ce3+ in compounds like LaCl3, LaBr3, Lu2SiO5, and Gd2SiO5 is utilized in scintillators for γ-ray detection.(Phosphor Handbook)
- Ce3+的跃迁发射能级的寿命与发射波长的平方成正比。(固体发光材料—P98)
取代格位:3+
3+
几种典型的Ce掺杂荧光粉:YAG掺杂的荧光粉,PLQY > 90%,Sr2.975-xBaxCe0.025AlO4F (PLQY ≈ 100%),Ca3Sc2Si3O12:Ce有high luminescence thermal stabilities with high PLQY。[Applied Materials Today-2020]
其他应用:
- 玻璃中的Ce
- CeO2用于玻璃脱色,玻璃中容易含有Fe2+使玻璃显现黄绿色,加入0.1% CeO2可将二价铁氧化为三价,从而大幅提高玻璃的透明度,用其替代过去使用的有毒的氧化砷。
- Ce玻璃还可以防紫外线、X射线和γ射线的辐照。因此用Ce玻璃可以制成防紫外的眼镜和电焊用的护目镜,也可以制成用来存储易被紫外光破坏的药物的玻璃容器。普通玻璃经γ射线辐照后会产生色心而着色,变为不透明,但加入二氧化铈后可防止玻璃的辐照变色,因而可用作原子能设施的观察窗。
注:稀土中的Sm、Eu、Gd、Dy具有很大的吸收慢中子和热中子的能力,可用 以制成吸收中子的防辐照玻璃,在原子能设施中使用。
- CMP (Chemical Mechanical Polishing):二氧化铈的另一大用途是作为玻璃的抛光粉。利用它抛光玻璃、眼镜片、照相机镜头和精密光学元件,可获得很低的粗糙度和高的抛光速度,远比过去使用的氧化铁抛光粉(红粉)优越。因此,氧化铈抛光粉是制备高级的光学镜头和元件所不可缺少的材料,使用量很大。参考:纳米氧化铈的在CMP抛光的应用
- 氧的调节剂—CeOx,当空气-燃料比小而缺氧时,CeOx放出氧;当氧过量时,它又吸收氧,从而起到了氧的调节剂的作用。空气和燃料比有一个最优值,此时转换器效率最高。
关于Ce4+的电荷迁移态发光,在大专题中有讨论,这里不赘述。
3+
- d-f 跃迁lifetimes of baout 10 μs(fast) due to \(\Delta S=0\)
- used in QC. The photon cascade emission involving the 4f2 levels of Pr3+ has been investigated for developing high quantum efficiency phosphors excited by means of a Xe discharge in the vacuum-UV. (Phosphor Handbook)
- low-lying 4f5d configuration
- four types of emission:
(1) red emission from \(^1D_2\) level to the ground.
(2) green emission from \(^3P_0\) level to the ground. - 发光特点还可以参见文献[Zoila Barandiaran-JPCL]
- 用于闪烁体,比如Pr3+掺杂的Lu3Al5O12 (LuAG)
For example, for scintillators materials in PET scanners, a short response time is crucial and it has been realized that the d-f emission of Pr3+ is faster than for the widely applied d-f emission from Ce3+. The shortest radiative lifetime that can be achieved for Pr3+ d-f emission is predicted to be ~ 6 ns. [Analysis of the radiative lifetime of Pr3+ df emission-JAP]
- infrared emission (900/ 1060 / 1350/ 1800 nm)
- yellow absorption
- 重要的laser ion

- Few applications.
- often lower efficiency, cross relaxations.
- Sm3+ is utilized as an efficient electron trap and much research has been devoted to its information storage properties. (Phosphor Handbook)
(1) MgS:Ce3+;Sm3+ and MgS:Eu2+;Sm3+ for optical memory phosphor applications.
(2) Y2SiO5:Ce3+;Sm3+ for X-ray imaging phosphor applications.
(3) LiYSiO4:Ce3+;Sm3+ for thermal neutron imaging phosphor applications. - 在锆钛酸铅压电功能陶瓷中加入氧化钐等稀土,可制成大功率超声换能器和压电点火 装置。
- Sm2+的红光可以用于horticultural application,参考[Exploring Sm2+ activated phosphors as red-deep red photon generator for horticultural application-2020]。
- Sm2+的压力下的发光,参考[Effect of pressure on energy levels of Sm2+ in BaFCl and SrFC—PRB-1995]。
- 容易形成Sm2+基质:很少有基质在高温和空气下Sm3+变为Sm2+,除了SrB4O7。大部分基质,即使using strong agent。文献[Sm2+ in Oxide Lattices: An Unexplored Chapter of Crystal Chemistry-1998]基于大量的实验,得出如下总结:
- host lattice should only provide M2+ sites,而且半径和Sm2+匹配;
- 基于xxx

- 其他可能的好文章:
电荷迁移带:
- 3+-O2-大;Eu3+的\(4f-4f\)跃迁,\(\Delta R\)小。其详细信息可以参考“大专题——荧光粉中电荷转移和价态变化-稀土和配位离子(O/F/Cl/Br/I/S)的电荷迁移态”。
- 在少数基质中3+-O2-
- 估算电荷迁移带的公式:Jørgensen公式,以及后续Krumpel的改进版。
[ECS-JSS-2016] - MO6基团有400 nm的宽带吸收,通过CTS将能量传递给Eu3+。[Philipe_JES_2011]
Eu3+发射峰的劈裂-和格位对称性:
- The presence of the 5D0→7F0 transition indicates that the Eu3+ could be located in one of the Cnv, Cn, or Cs symmetry group. 如果Eu3+占据的格位有inversion center,那么就没有Eu3+的5D0→7F0的跃迁。只观察到一个5D0到7F0的跃迁峰,不能说明只有一个占据格位,因为可能存在一个有对称中心的格位。5D0到7F0的跃迁,两个能级的J值都是零(2J + 1 = 1),不存在劈裂(no stark effect),如果是跃迁到7F2,那么最多存在5个劈裂(2J + 1 = 5),如果J = 1最多有三个劈裂(2J + 1 = 3)。
- stark effect results in sublevels of the ground-state and excited-state manifolds.
- Eu3+的5D0→7FJ (J = 0-4)在不同point group下的劈裂数目可以参考Table-8 in [Interpretation of europium(III) spectra-Koen Binnemans-Coordination Chemistry Reviews-2015],或者也可以看看[Application of the Eu3+ ion for site symmetry determination-Journal of Rare Earths-1996]
- 5D0→7F1 / 5D0→7F2的比值不一定够能用来对比两个格位的对称性相对大小。
参考[Some misconceptions concerning the electronic spectra of tri-positive europium and cerium-Chemical Society Reviews-2013] - 5D0→7F0的跃迁来探测局域环境,比如, in Y2SiO5 material, Eu3+ was known to occupy the Y3+ location at two different types of site, while it is found that Eu3+ occupies numerous distinct sites in this material.
参考[Site-selective hole burning in Eu3+:Y2SiO5] - 5D0→7F0发光最强的例子参考这里。
- Eu3+掺杂导致的Breakdown of Crystallographic Site Symmetry (即从所谓的高对称性变为低对称性),NaYF4中存在disorder,即two or more cations statistically occupy the same lattice site. Because the microscopic model of such structural disorder is established from a least-squares fitting of single-crystal X-ray diffraction (XRD) data by standard crystallography analysis, which takes into account those cations randomly occupying the same lattice site as a virtual “average” ion with their respective probabilities, the actual local symmetry of the dopant in the disorder site can not be revealed from the crystallographic data.
参考[Breakdown of Crystallographic Site Symmetry in Lanthanide-Doped NaYF4 Crystals-Angew-2012] 新闻介绍链接

四种用途:
- a probe for structural evaluation
- 高效红色荧光粉
- 防伪
- Eu3+的螯合物,用于时间分辨荧光免疫测定
Eu3+含量的探测-XANES:
- 有Eu3+的XANES信号,但是没有测到其PL,作者认为Eu3+的XANES信号is possibly because of the unreacted starting material Eu2O3 and sample surface oxidation.
[Wang, Meng, et al. "Eu sites in Eu-doped AlON phosphors: anomalous Eu occupancy layers." The Journal of Physical Chemistry C 123.5 (2019): 3110-3114.] - 作者在其样品中观察到Eu2+的发光占主导(即使激发Eu3+的CT band),但是XANES信号表明Eu3+的含量比Eu2+更高。A considerable fraction of Eu3+ ions can be present in the powders, although this is not necessarily reflected in the photoluminescence properties.
[Valence states of europium in CaAl2O4:Eu phosphors-OME-2012] - 还原气氛下合成的Li4SrCa(SiO4)2:Eu2+主要是Eu2+发光,但是XANES测试表明Eu2+只占很小的比例,主要是Eu3+的XANES信号。[Li4SrCa(SiO4)2:Eu2+: A Potential Temperature Sensor—2022]
- 还原气氛下合成的SrLiAl3N4:Eu2+,没有测到Eu3+的发光,XANES测试表明Eu2+只占很小的比例,主要是Eu3+的XANES信号。This result may be due to the possibility that the electron located at the excited state of Eu3+ underwent a nonradiative transition to ground state because lattice relaxation diminished the energy of charge transfer state below the energy of 5D0 state regardless of whether higher or lower energy was chosen as the excitation wavelength.
- 可能是如果host里面含有Li,就容易形成Eu3+,即使是还原气氛,其他例子还有Li2SrSiO4、Li2CaSiO4、LiSrPO4等。
- 3:Eu2+
- 类似Y3Ga5O12: Ce3+
- 宽带激发谱没有特征:
(1) 5d excited state is split by the crystal field into two or more components, depending on the local symmetry. Each corresponding absorption band is rather broad (typically 4000 cm-1), due to the 4f6(7FJ) electronic level structure. The excitation spectrum is rather featureless due to the partially overlapping excitation bands. [] (2) M. Suta发表在JL的文章表面上正好指认了7Fj的不同能级,实际上这只是巧合,真实的情况是4f6电子肯定会和5d电子有相互作用,这样的话7Fj这种notation本身就是错误。
- 确定Eu2+的浓度:A Non-Destructive Method for Determining the Eu2+ Concentration in the Alkali Chlorides
- 格位:The phenomenon of one crystallographic site, but multiple local environments, was also observed in the BaScO2F:Eu2+ perovskite and Sr3La(PO4)3:Eu2+ eulytite phosphors.
(1)The element europium was named after the continent of Europe.
(2) Europium is primarily obtained through an ion exchange process from monazite sand ((Ce,La,Th,Nd,Y)PO4 ), a material rich in rare earth elements.
(3) A soft, silvery metal that tarnishes quickly and reacts with water.
(4) Eu2+ and Eu3+ are strong paramagnetic ions (6 or 7 unpaired 4f electrons)
(5) Eu as a marker for anticounterfeiting
(6) Eu2+ afterglow phopshor
(7) Europium – Further Applications: MRT Contrast enhancement / 151Eu Mößbauer spectroscopy / Alloys / Neutron absorber / Scintillators / Storage phosphors, imaging plate: Ba(F,Br):Eu
以上内容来自FH Münster-International Year of the Periodic Table (IYPT)-Europium
Eu2+-Eu3+共掺杂的应用
(1) 热分解,原位还原,oleylamine 作为还原剂,通过控制温度,控制还原的比例(参考Pan Y, Xie X, Huang Q, et al. Inherently Eu2+/Eu3+ Codoped Sc2O3 Nanoparticles as High‐Performance Nanothermometers[J]. Advanced Materials, 2018, 30(14): 1705256.) 还有好几篇类似的文章,这里不赘述;
(2) 防伪,Eu2+/Eu3+-Based Smart Duplicate Responsive Stimuli and Time-gated Nanohybrid for Optical Recording and Encryption
(3) 白光LED,
Gautier R, Li X, Xia Z, et al. Two-step design of a single-doped white phosphor with high color rendering[J]. Journal of the American Chemical Society, 2017, 139(4): 1436-1439.
Liu X, Xie W, Lu Y, et al. Multichannel luminescence properties of mixed-valent Eu2+/Eu3+ coactivated SrAl3BO7 nanocrystalline phosphors for near-UV LEDs[J]. Inorganic Chemistry, 2017, 56(22): 13829-13841.
- 4f最低激发能级是6P7/2,因此其发射光谱线最为简单,通常仅有6P7/2-8S7/2跃迁所对应的的~ 315 nm谱线,这种跃迁只有在有高能光吸收的基质晶格中才能观察到,可作为敏化剂。
- 虽然其电子组态和Eu2+相同,但是其4f65d态处于相当高的能态,因而观察不到其跃迁发射。
- 磁共振成像的反差剂(含钆(Gd3+)的顺磁配合物),医疗用的磁共振成像系统是测氢核(1H)的磁共振的,它的成像强度取决于氢核(1H)的弛豫时间。人体内含有大量的水(H2O),因此含有大量的氢核和质子。上述的含钆的磁共振成像反差剂可通过跟其周围的氢核(1H)的相互作用,催化水的质子的弛豫,从而可加速获得图像,并可提高信号强度,增强图像的反差,便于区别正常的和异常病变的组织与器官而有利于医生的诊断。(参考:为什么大部分MR造影剂都是Gd基的?)
- medium efficiency, limited by cross relaxations
- poor color rendering
- unsuitable wavelength for display.
- Dy3+ plays an important role in the persistent luminescence phosphor SrAl2O4:Eu2+;Dy3+.
- green emission and red emission.
- Er3+ and Tm3+ are, like Pr3+, investigated for possible photon cascade emission phosphor applications.
- 1550 nm in optical fiber matches with low losses in silica fiber (EDFA)
2+/Tm
- 2+, Sm2+等来说,Tm2+更难稳定存在,因为Tm3+/Tm2+的reduction potential负得更多,大部分对于Tm2+的研究集中在单晶。研究发现Tm2+材料可作为luminescent solar concentrators, due to their outstanding broadband absorption and large stokes shift, which minminzes self-absorption. [
- 2+是excellent up-conversion activator. So X-RAY convert Tm3+ to Tm2+, then up-conversion intensity of Tm3+ can be the indicator of X-RAY dose. [
- 2+
- Unfolding the Excited States Dynamics of Tm2+-doped Halides-PHD
- 13
- infrared laser 980 nm
- for UC/QC
- CTS emission possible (两个发射峰) [Charge transfer luminescence of Yb3+-JL-2000] [Phosphor Handbook]
- Yb原子钟
Yb3+具有Quasi-three-level Characteristics,可以作为laser-active dopant。虽然Yb3+只有一个excited state manifold(2F5/2)以及一个ground-state manifold(2F7/2),但是Pumping and amplification involve transitions between different sublevels of the ground-state and excited-state manifolds. The sublevels would be energy-degenerate in vacuum, but that degeneracy is removed by the electric field in the crystal lattice.
比如CaF:Yb3+激光晶体,还有YAG:Yb3+。
Yb3+因其简单的二能级结构,不存在激发态吸收和交叉弛豫等多余的非辐射跃迁而具有高的能量转换效率和量子效率等优势。Yb3+的主要优点是Low quantum defect , long fluorescence lifetime。【YDF】(Yb3+掺杂光纤)采用易于获得高功率激光输出,掺Yb3+光纤也在高功率光纤激光器应用中占据了半壁江山。可能存在的问题是光暗化。
参考资料:
(1) Ytterbium-doped Laser Gain Media—rp-photonics
(2) 三能级激光器相比于四能级激光器有什么优点?-为什么是Yb
(3) 为什么大多数连续光纤激光器的波长是1080nm,而大多数脉冲激光器的波长却是1064nm?
(4) 掺镱光纤光暗化研究进展-胡丽丽
(5) 掺镱光纤激光器光子暗化现象的研究进展
(6) 掺Yb3+光纤中光暗化的研究概述-2015
(7) 掺Yb3+激光晶体的研究进展
- 在很多基质中没有发光,因为interaction of the 5d excited state with the conduction band levels。
- 在某些基质中表现出anomalous emission,即斯托克斯位移很大,而且发射峰很宽,这被认为formation of a self-trapped exciton 这个发光看起来interesting for LED applications,但是由于strong thermal quenching,所以不实用。
- 2+[Philipe_JES_2011]
- 2+
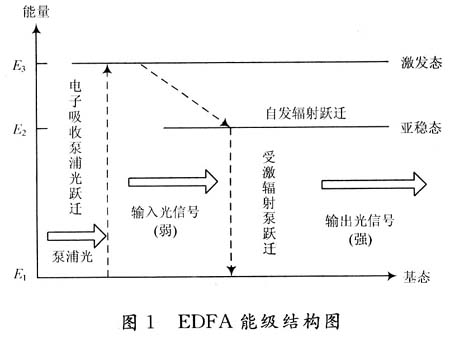
- It is an interesting lanthanide ion because it has the same excitation/emission range as divalent europium, while at the same time its excitation spectrum yields much more information than that of Eu2+ in the same host. [Luminescence of ytterbium in CaS and SrS-JL-2014] 其实就是vibronic structure(低温)
- relatively long decay time(1- 10 ms),所以在高flux辐照下有明显的saturation effect。