CaS材料
Eu,Sm concentration on PL and OSL
[Study on relationship of luminescence in CaS:Eu,Sm and dopants concentration-JL-2002]
摘要: CaS:Eu,Sm是 promising candidate for electron trapping optical memory materials, rock-salt crystalline structure。Eu2+ acts as luminescent centre while Sm3+ acts as electron trap site. 从应用的角度看,高OSL效率很关键。Eu/Sm的掺杂浓度对PL/OSL的影响需要详细研究。
单掺杂的PLE
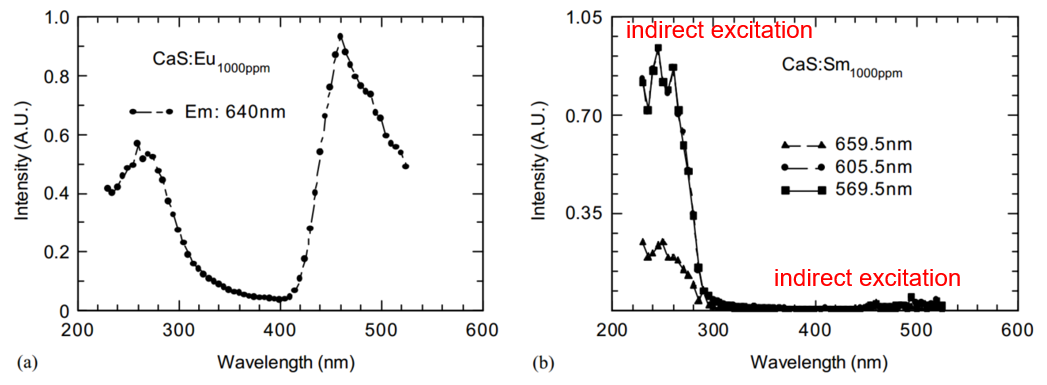
460 nm激发下的delayed PL

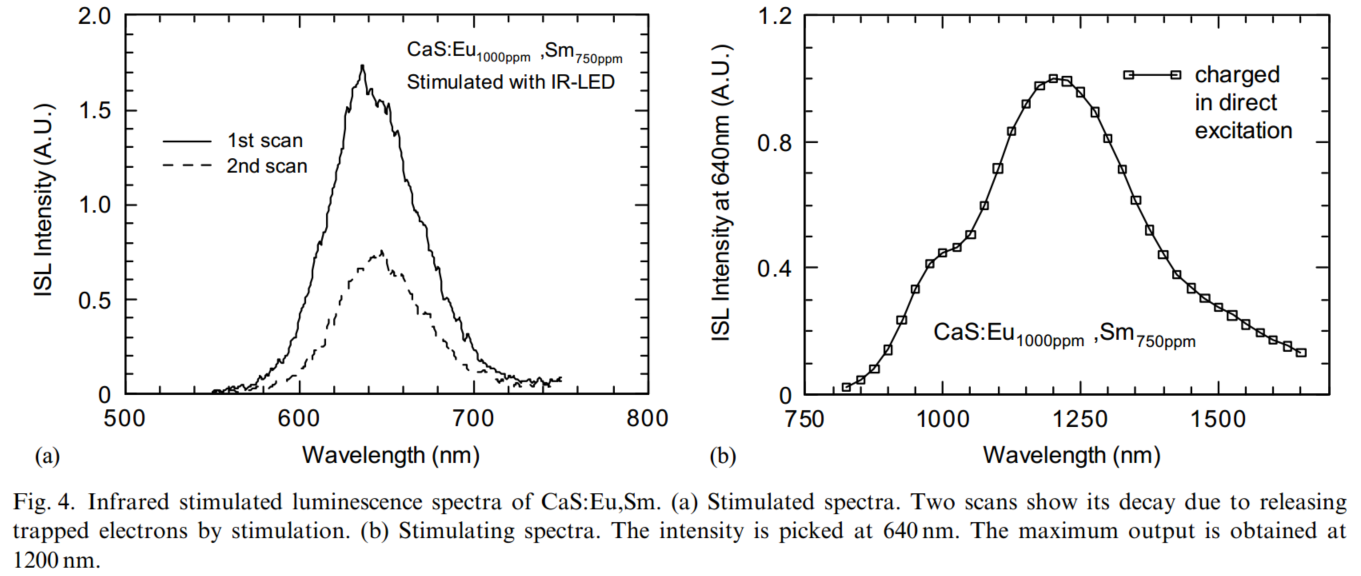
OSL的测试:charged in direct excitation, then kept in dark to eliminate after-glow influence, 最后再测OSL。
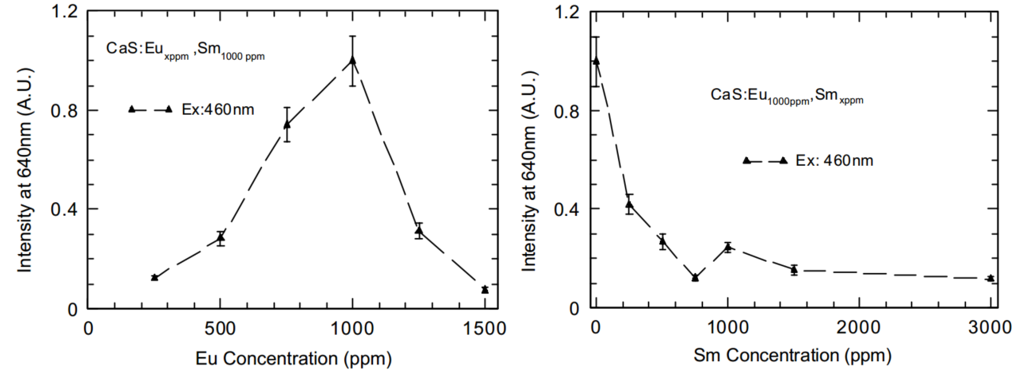
PL versus Eu/Sm concentration
(1) at above 1000 ppm of Eu, not all Eu in CaS:Eu,Sm play as luminescence centres.
(2) trapping sites are increased with increment of Sm concentration, which reduces the PL of Eu2+.
(3) abruptly increases with further increment of Sm above 750 ppm, means not all Sm play as electron trapping centres. More Sm reduces the number of trapping sites. Therefore, we cannot increase Sm concentration to increase the electron trapping sites any further at high Sm concentration above 750 ppm.
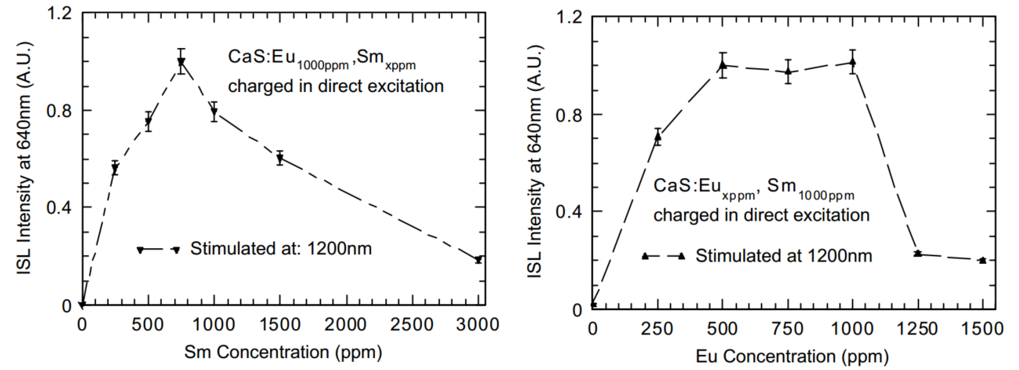
OSL versus Eu/Sm concentration
(1) Sm的浓度也是超过750 ppm后OSL效果不好了,这和之前PL的结果一致,说明继续增加Sm的浓度并没有增加trapping sites的数量。
(2) 固定Sm的浓度,然后变化Eu的浓度,the emission intensity in ISL increases with the increment of the concentration of Eu up to 500 ppm as there are more luminescence centres of Eu2+ to be ionized。
讨论和结论
- 现象:低掺杂浓度下,Eu2+作为PL center,Sm3+作为trapping center都很好,但是一旦超过一定浓度,more dopants will reduce the number of luminescence centres and electron trapping sites.
- 解释:ISL occurs due to electronic transitions between two dopants. Consequently, ISL is determined by homogeneous distribution of not only Eu and Sm in CaS but also between Eu and Sm. 低掺杂浓度下确实可以实现 homogeneous distribution,因为Ca2+和Eu2+的半径/电荷差不多。随着Eu浓度到达一定数量,they become closer and closer to each other. Those closely positioned Eu2+ will change their local electronic field and tend to aggregate with each other. Finally, a cluster of Eu2+ is formed in CaS which prevents Sm3+ replacing adjacent Ca2+. Therefore, both PL and ISL drop down at high level of Eu.
- ISL的复杂性:Sm3+需要电荷平衡,所以其局域环境比Eu2+更复杂,这可能也解释了为啥Sm3+的f-f激发那么弱,发光vanish那么慢。